Spatial Patterns of Endometriosis Incidence. A Study in Friuli Venezia Giulia (Italy) in the Period 2004–2017
Abstract
:1. Introduction
2. Materials
2.1. Incidence of Endometriosis
- At least one hospitalization with a diagnosis of endometriosis associated with the identification of endometriosis from the anatomic pathology database. The association was via a unique anonymous identifier of women and temporal proximity.
- At least one hospitalization with a diagnosis of endometriosis confirmed by laparoscopy or a surgical procedure allowing direct visualization.
- Identification of endometriosis from anatomic pathology database, without hospitalization with a diagnosis of endometriosis.
2.2. Standardized Incidence Ratios
3. Methods
3.1. Models for Disease Mapping
3.2. Profiling Municipalities at High Risk
3.2.1. Simple Approaches Based on p-values
3.2.2. Hierarchical Bayesian Mixture Model
3.3. Sensitivity Analysis
4. Results
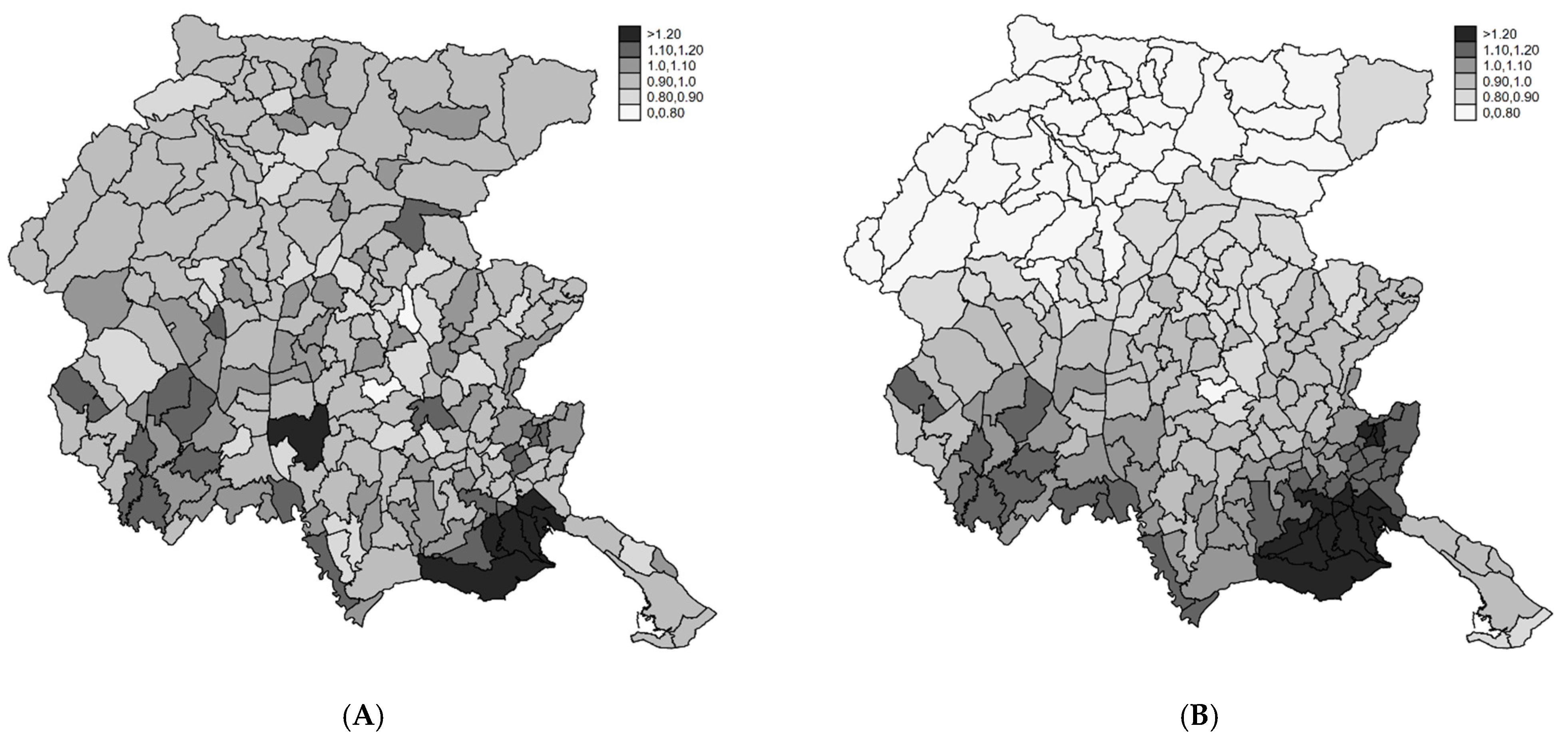
4.1. Disease Mapping
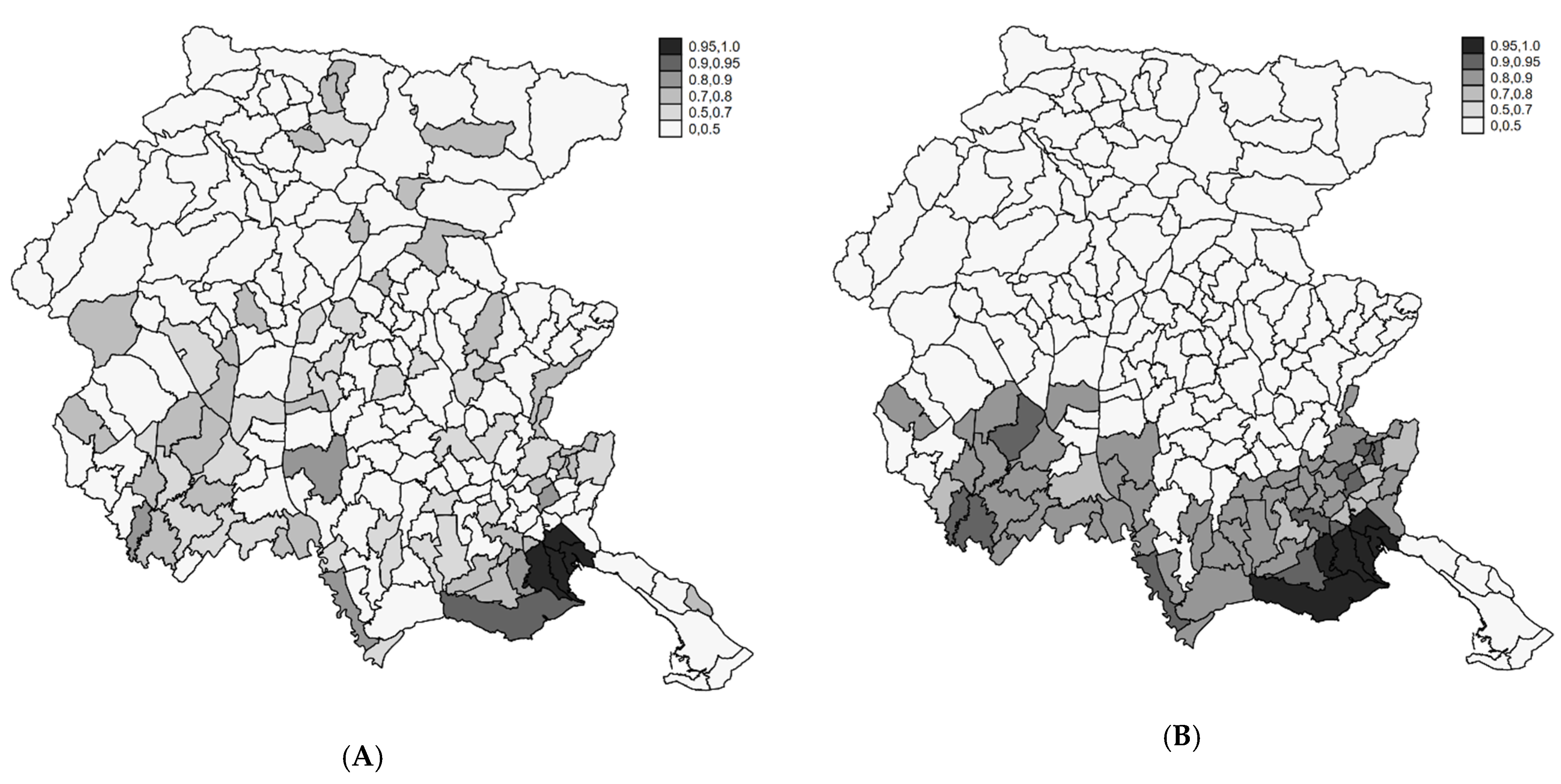
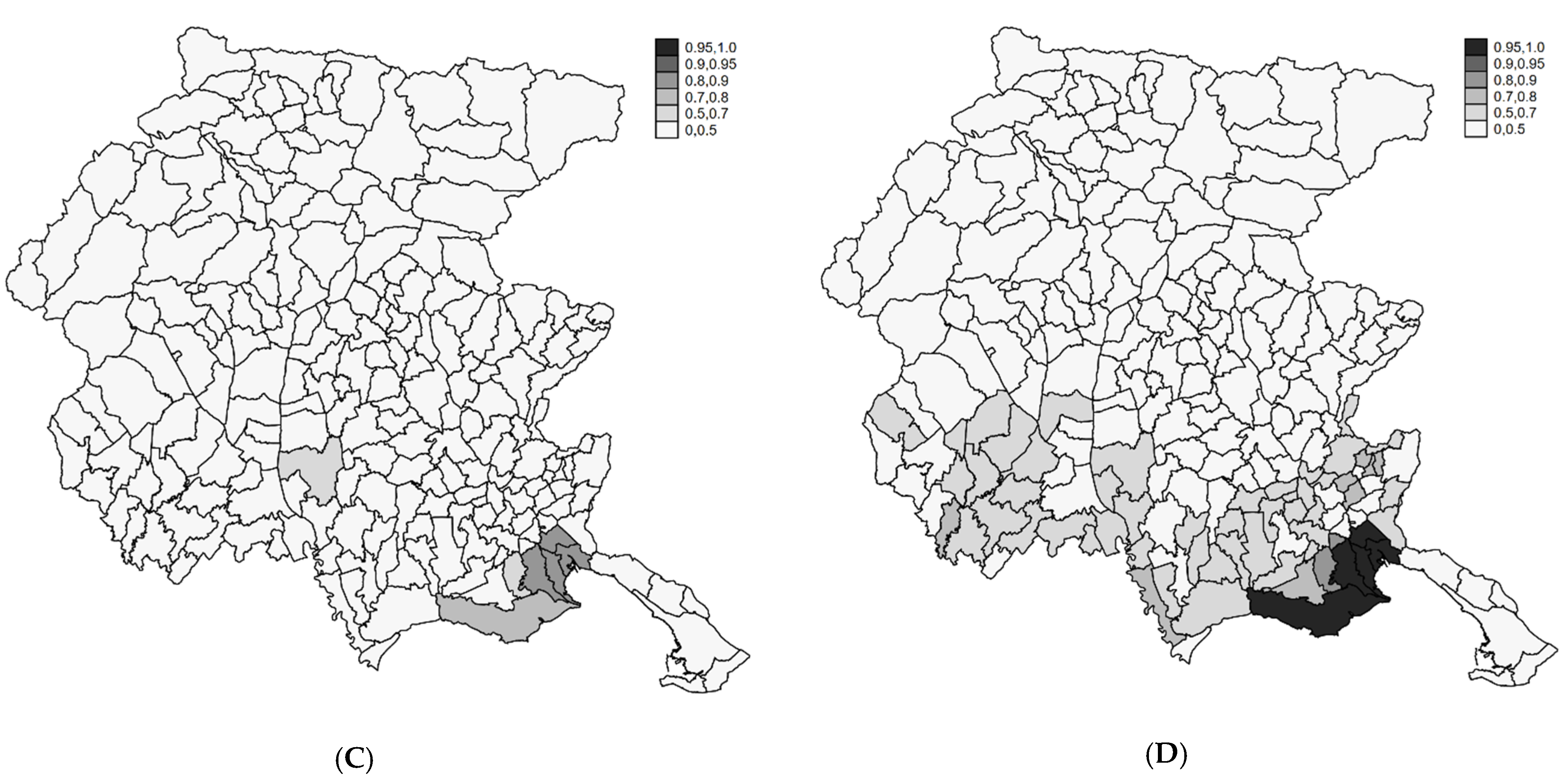
4.2. Profiling Municipalities at High Risk
4.3. Sensitivity Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Valbrun, T.G.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef]
- Leibson, C.L.; Good, A.E.; Hass, S.L.; Ransom, J.; Yawn, B.P.; O’Fallon, W.M.; Melton, L.J. Incidence and characterization of diagnosed endometriosis in a geographically defined population. Fertil. Steril. 2004, 82, 314–321. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; Study, W.E. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [Green Version]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- Rogers, P.A.; D’Hooghe, T.M.; Fazleabas, A.; Gargett, C.E.; Giudice, L.C.; Montgomery, G.W.; Rombauts, L.; Salamonsen, L.A.; Zondervan, K.T. Priorities for endometriosis research: Recommendations from an international consensus workshop. Reprod. Sci. 2009, 16, 335–346. [Google Scholar] [CrossRef]
- Hsu, A.L.; Khachikyan, I.; Stratton, P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin. Obstet. Gynecol. 2010, 53, 413–419. [Google Scholar] [CrossRef]
- Gylfason, J.T.; Kristjansson, K.A.; Sverrisdottir, G.; Jonsdottir, K.; Rafnsson, V.; Geirsson, R.T. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am. J. Epidemiol. 2010, 172, 237–243. [Google Scholar] [CrossRef]
- Ferrero, S.; Arena, E.; Morando, A.; Remorgida, V. Prevalence of newly diagnosed endometriosis in women attending the general practitioner. Int. J. Gynaecol. Obstet. 2010, 110, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Migliaretti, G.; Deltetto, F.; Delpiano, E.M.; Bonino, L.; Berchialla, P.; Dalmasso, P.; Cavallo, F.; Camanni, M. Spatial Analysis of the Distribution of Endometriosis in Northwestern Italy. Gynecol. Obstet. Invest. 2012, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.E.; Noller, K.L.; Melton, L.J., 3rd; Selwyn, B.J.; Hardy, R.J. Incidence of pelvic endometriosis in Rochester, Minnesota, 1970-1979. Am. J. Epidemiol. 1987, 125, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Hediger, M.L.; Peterson, C.M.; Croughan, M.; Sundaram, R.; Stanford, J.; Chen, Z.; Fujimoto, V.Y.; Varner, M.W.; Trumble, A.; et al. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil Steril. 2011, 96, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Hummelshoj, L.; Prentice, A.; Groothuis, P. Update on endometriosis. Womens Health (Lond) 2006, 2, 53–56. [Google Scholar] [CrossRef]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Marshall, L.M.; Hunter, D.J. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 2004, 160, 784–796. [Google Scholar] [CrossRef]
- Morassutto, C.; Monasta, L.; Ricci, G.; Barbone, F.; Ronfani, L. Incidence and Estimated Prevalence of Endometriosis and Adenomyosis in Northeast Italy: A Data Linkage Study. PLoS ONE 2016, 11, e0154227. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.; Thomson, A.; Best, N.; Elliott, P. Interpreting posterior relative risk estimates in disease-mapping studies. Environ. Health Perspect. 2004, 112, 1016–1025. [Google Scholar] [CrossRef]
- Storey, J.D. The positive false discovery rate: A Bayesian Interpretation and the Q-Value. Ann. Statist. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Wakefield, J. Reporting and interpretation in genome wide association studies. Int. J. Epidemiol. 2008, 37, 641–653. [Google Scholar] [CrossRef] [Green Version]
- Catelan, D.; Biggeri, A. Multiple testing in descriptive epidemiology. Geospat. Health 2010, 4, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Best, N.; Richardson, S.; Thomson, A. A comparison of Bayesian spatial models for disease mapping. Stat. Methods Med. Res. 2005, 14, 35–59. [Google Scholar] [CrossRef]
- Militino, A.F.; Ugarte, M.D.; Dean, C.B. The use of mixture models for identifying high risks in disease mapping. Stat. Med. 2001, 20, 2035–2049. [Google Scholar] [CrossRef]
- Biggeri, A.; Marchi, M.; Lagazio, C.; Martuzzi, M.; Böhning, D. Non parametric maximum likelihood estimators for disease mapping. Stat. Med. 2000, 19, 2539–2554. [Google Scholar] [CrossRef]
- Muller, P.; Parmigiani, G.; Rice, K. FDR and Bayesian Multiple Comparisons Rules. In Proceedings of the Proc Valencia 2007/ISBA 8th World Meeting on Bayesian Statistics, Benidorm-Alicante, Spain, 1–6 June 2006; Oxford University Press: Oxford, UK. [Google Scholar]
- Catelan, D.; Lagazio, C.; Biggeri, A. A hierarchical Bayesian approach to multiple testing in disease Mapping. Biom. J. 2010, 52, 784–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISTAT: The National Institute of Statistics. Available online: http://www.demo.istat.it (accessed on 24 November 2020).
- Breslow, N.E.; Day, N.E. Statistical Methods in Cancer Research—Volume II—The Design and Analysis of Cohort Studies; IARC Scientific Publications; Oxford University Press: Oxford, UK, 1987; Volume 82. [Google Scholar]
- Clayton, D.; Kaldor, J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics 1987, 43, 671–681. [Google Scholar] [CrossRef]
- Besag, J.; York, J.C.; Molliè, A. A Bayesian image restoration, with two applications in spatial statistics (with discussion). Ann. Inst. Statist. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Besag, J.; Kooperberg, G. On conditional and intrinsic autoregressions. Biometrika 1995, 82, 733–746. [Google Scholar]
- Best, N.G.; Arnold, R.A.; Thomas, A.; Waller, L.A.; Conlon, E.M. Bayesian models for spatially correlated disease and exposure data. In Bayesian Statistics 6; Bernardo, J.M., Berger, J.O., Dawid, A.P., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 131–156. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R Statist. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Jones, H.E.; Ohlssen, D.I.; Spiegelhalter, D.J. Use of the false discovery rate when comparing multiple health care providers. J. Clin. Epidemiol. 2008, 61, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.N. Of P-Values and Bayes: A Modest Proposal. Epidemiology 2001, 12, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.; Lindman, H.; Savage, L.J. Bayesian statistical inference in psychological research. Psychol. Rev. 1963, 70, 193–242. [Google Scholar] [CrossRef]
- Sellke, T.; Bayarri, M.J.; Berger, J.O. Calibration of p values for testing precise null hypotheses. Am. Stat. 2001, 55, 62–71. [Google Scholar] [CrossRef]
- Scott, J.G.; Berger, J.O. An Exploration of Aspects of Bayesian Multiple Testing. J. Stat. Plan. Inference 2006, 136, 2144–2162. [Google Scholar] [CrossRef]
- Barbieri, M.M.; Berger, J.O. Optimal Predictive Model Selection. Ann. Stat. 2004, 32, 870–897. [Google Scholar] [CrossRef]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–511. [Google Scholar] [CrossRef]
- Chao, A.; Tsay, P.K.; Lin, S.H.; Shau, W.Y.; Chao, D.Y. The applications of capture-recapture models to epidemiological data. Stat. Med. 2001, 20, 3123–3157. [Google Scholar] [CrossRef]
- Egger, M.; Davey-Smith, G.; Altman, D. Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed.; BMJ Pub. Group: London, UK, 2001. [Google Scholar]
- Hyunkyung, K.; Minkyoung, L.; Hyejin, H.; Chung, Y.J.; Cho, H.H.; Yoon, H.; Kim, M.; Chae, K.H.; Jung, C.Y.; Kim, S.; et al. The Estimated Prevalence and Incidence of Endometriosis with the Korean National Health Insurance Service-National Sample Cohort (NHIS-NCS): A National Population-Based Study. J. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Rowlands, I.J.; Abbott, J.A.; Montgomery, G.W.; Hockey, R.; Rogers, P.; Mishra, G.D. Prevalence and Incidence of Endometriosis in Australian Women: A Data Linkage Cohort Study. BJOG 2020. [Google Scholar] [CrossRef]
- Editorial: SMMR Special issue on disease mapping. Stat. Methods Med. Res. 2005, 14, 1–2. [CrossRef] [Green Version]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Roncella, E.; Cipriani, S.; Trojano, G.; Barbera, V.; Herranz, B.; Colli, E. The frequency of endometriosis in the general and selected populations: A systematic review. JEPPD 2020, 12, 176–189. [Google Scholar] [CrossRef]
- Ghiasi, M.; Kulkarni, M.T.; Missmer, S.A. Is Endometriosis More Common and More Severe Than It Was 30 Years Ago? J. Minim. Invasive Gynecol. 2020, 27, 452–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernuit, D.; Ebert, A.D.; Halis, G.; Strothmann, A.; Gerlinger, C.; Geppert, K.; Faustmann, T. Female perspectives on endometriosis: Findings from the uterine bleeding and pain women’s research study. J. Endometr. 2011, 3, 73–85. [Google Scholar] [CrossRef]
- Chapron, C.; Lang, J.H.; Leng, J.H.; Zhou, Y.; Zhang, X.; Xue, M.; Popov, A.; Romanov, V.; Maisonobe, P.; Cabri, P. Factors and Regional Differences Associated with Endometriosis: A Multi-Country, Case-Control Study. Adv. Ther. 2016, 33, 1385–1407. [Google Scholar] [CrossRef] [Green Version]
- Buck Louis, G.M.; Chen, Z.; Peterson, C.M.; Hediger, M.L.; Croughan, M.S.; Sundaram, R.; Stanford, J.B.; Varner, M.W.; Fujimoto, V.Y.; Giudice, L.C.; et al. Persistent lipophilic environmental chemicals and endometriosis: The ENDO Study. Environ. Health Perspect. 2012, 120, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Von Theobald, P.; Cottenet, J.; Iacobelli, S.; Quantin, C. Epidemiology of Endometriosis in France: A Large, Nation-Wide Study Based on Hospital Discharge Data. Biomed Res. Int. 2016, 2016, 3260952. [Google Scholar] [CrossRef] [Green Version]



| Age | Women Residing in the Region * | Endometriosis n (Rates × 105) |
|---|---|---|
| 15–20 | 402,526 | 48 (12) |
| 21–25 | 365,815 | 228 (62) |
| 26–30 | 442,259 | 631 (143) |
| 31–35 | 541,962 | 869 (160) |
| 36–40 | 633,878 | 846 (133) |
| 41–45 | 681,123 | 833 (122) |
| 46–50 | 654,892 | 670 (102) |
| total 15–50 | 3,722,455 | 4125 (111) |
| Decrease in Probability of the Null Hypothesis | |||||||
|---|---|---|---|---|---|---|---|
| Municipality | Number of Cases | SIR a | p-Value | q-Value | From 75% to No Less than | From 50% to No Less than | From 25% to No Less than |
| San Canzian d’Isonzo | 38 | 1.759 | 0.0005 | 0.0522 | 0.0127 | 0.0043 | 0.0014 |
| Staranzano | 41 | 1.720 | 0.0005 | 0.0522 | 0.0128 | 0.0043 | 0.0014 |
| Ronchi dei Legionari | 62 | 1.498 | 0.0011 | 0.0640 | 0.0260 | 0.0088 | 0.0030 |
| Monfalcone | 114 | 1.339 | 0.0012 | 0.0640 | 0.0287 | 0.0098 | 0.0033 |
| Grado | 41 | 1.595 | 0.0019 | 0.0836 | 0.0442 | 0.0152 | 0.0051 |
| Lusevera | 6 | 2.986 | 0.0046 | 0.1672 | 0.0924 | 0.0328 | 0.0112 |
| San Lorenzo Isontino | 11 | 2.073 | 0.0085 | 0.2619 | 0.1480 | 0.0547 | 0.0189 |
| Fiumicello | 26 | 1.565 | 0.0117 | 0.2858 | 0.1864 | 0.0709 | 0.0248 |
| Codroipo | 72 | 1.307 | 0.0119 | 0.2858 | 0.1892 | 0.0722 | 0.0253 |
| Mariano del Friuli | 10 | 1.998 | 0.0138 | 0.2982 | 0.2096 | 0.0812 | 0.0286 |
| Morsano al Tagliamento | 15 | 1.697 | 0.0190 | 0.3253 | 0.2587 | 0.1042 | 0.0373 |
| Latisana | 64 | 1.295 | 0.0191 | 0.3253 | 0.2593 | 0.1045 | 0.0374 |
| Gradisca d’Isonzo | 31 | 1.438 | 0.0209 | 0.3253 | 0.2743 | 0.1119 | 0.0403 |
| Capriva del Friuli | 11 | 1.798 | 0.0229 | 0.3253 | 0.2900 | 0.1198 | 0.0434 |
| Turriaco | 16 | 1.624 | 0.0239 | 0.3253 | 0.2977 | 0.1238 | 0.0450 |
| Prata di Pordenone | 42 | 1.350 | 0.0249 | 0.3253 | 0.3047 | 0.1275 | 0.0464 |
| Barcis | 2 | 3.203 | 0.0256 | 0.3253 | 0.3096 | 0.1300 | 0.0475 |
| Polcenigo | 17 | 1.557 | 0.0303 | 0.3528 | 0.3401 | 0.1466 | 0.0542 |
| Cordenons | 78 | 1.233 | 0.0310 | 0.3528 | 0.3448 | 0.1492 | 0.0552 |
| Fiume Veneto | 53 | 1.276 | 0.0359 | 0.3865 | 0.3726 | 0.1652 | 0.0619 |
| Arba | 8 | 1.801 | 0.0376 | 0.3865 | 0.3811 | 0.1703 | 0.0640 |
| Dolegna del Collio | 3 | 2.378 | 0.0394 | 0.3865 | 0.3901 | 0.1758 | 0.0664 |
| Mossa | 9 | 1.689 | 0.0454 | 0.4263 | 0.4179 | 0.1931 | 0.0739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catelan, D.; Giangreco, M.; Biggeri, A.; Barbone, F.; Monasta, L.; Ricci, G.; Romano, F.; Rosolen, V.; Zito, G.; Ronfani, L. Spatial Patterns of Endometriosis Incidence. A Study in Friuli Venezia Giulia (Italy) in the Period 2004–2017. Int. J. Environ. Res. Public Health 2021, 18, 7175. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18137175
Catelan D, Giangreco M, Biggeri A, Barbone F, Monasta L, Ricci G, Romano F, Rosolen V, Zito G, Ronfani L. Spatial Patterns of Endometriosis Incidence. A Study in Friuli Venezia Giulia (Italy) in the Period 2004–2017. International Journal of Environmental Research and Public Health. 2021; 18(13):7175. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18137175
Chicago/Turabian StyleCatelan, Dolores, Manuela Giangreco, Annibale Biggeri, Fabio Barbone, Lorenzo Monasta, Giuseppe Ricci, Federico Romano, Valentina Rosolen, Gabriella Zito, and Luca Ronfani. 2021. "Spatial Patterns of Endometriosis Incidence. A Study in Friuli Venezia Giulia (Italy) in the Period 2004–2017" International Journal of Environmental Research and Public Health 18, no. 13: 7175. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18137175







