Effect of Sanitation Interventions on Health Outcomes: A Systematic Review of Cluster-Randomized Controlled Trials in Rural Communities of Low- and Middle-Income Countries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy, Inclusion Criteria and Data Extraction
2.2. Assessment and Analysis of Included Studies
3. Results
3.1. Characteristics of Included studies
3.1.1. Characteristics of Participants
3.1.2. Intervention, Adherence, Latrine Coverage, and Attrition at Follow-Up
3.1.3. Subsidies, Sanitation Demand, and Intention-to-Treat
3.1.4. Risk of Bias Assessment
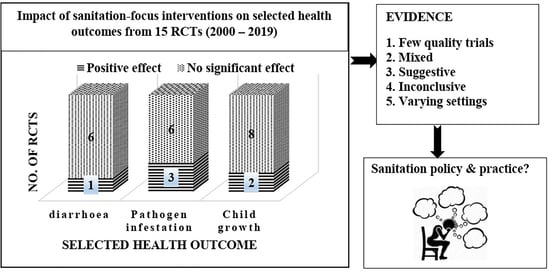
3.2. Health Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prüss-Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M.; Cumming, O.; Curtis, V.; Bonjour, S.; Dangour, A.D.; de France, J.; Fewtrell, L.; et al. Burden of diseases from inadequate water, sanitation and hygiene in low- and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R.; et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef]
- Clasen, T.; Prüss-Ustün, A.; Mathers, C.D.; Cumming, O.; Cairncross, S.; Colford, J.M., Jr. Estimating the impact of unsafe water, sanitation and hygiene on the global burden of disease: Evolving and alternative methods. Trop. Med. Int. Health 2014, 19, 884–893. [Google Scholar] [CrossRef]
- World Health Organisation/United Nations Children’s Fund (WHO/UNICEF). Progress on Sanitation and Drinking Water - 2015 Update and MDG Assessment. World Health Organisation. 2015. Available online: http://files.unicef.org/publications/files/Progress_on_Sanitation_and_Drinking_Water_2015_Update_.pdf (accessed on 16 January 2020).
- Fewtrell, L.; Colford, J.M. Water, sanitation and hygiene in developing countries: Interventions and diarrhea—A review. Water Sci. Technol. 2005, 52, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Prüss-Ustün, A.; Cumming, O.; Bartram, J.; Bonjour, S.; Cairncross, S.; Clasen, T.; Colford, J.M., Jr.; Curtis, V.; De France, J.; et al. Systematic review: Assessing the impact of drinking water and sanitation on diarrhoeal disease in low- and middle-income settings: Systematic review and meta-regression. Trop. Med. Int. Health 2014, 19, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Mara, D.; Lane, J.; Scott, B.; Trouba, D. Sanitation and Health. PLoS Med. 2010, 7, e1000363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvesh, N.; Das, J.K.; Vaivada, T.; Gaffey, M.F.; Rasanathan, K.; Bhutta, Z.A.; Social Determinants of Health Study Team. Water, sanitation and hygiene interventions for acute childhood diarrhea: A systematic review to provide estimates for the Lives Saved Tool. BMC Public Health 2017, 17, 776. [Google Scholar] [CrossRef]
- Wolf, J.; Hunter, P.; Freeman, M.C.; Cumming, O.; Clasen, T.; Bartram, J.; Higgins, J.; Johnston, R.; Medlicott, K.; Boisson, S.; et al. Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: Updated meta-analysis and meta-regression. Trop. Med. Int. Health 2018, 23, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Ziegelbauer, K.; Speich, B.; Mäusezahl, D.; Bos, R.; Keiser, J.; Utzinger, J. Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-Analysis. PLoS Med. 2012, 9, e1001162. [Google Scholar] [CrossRef] [Green Version]
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, Sanitation, Hygiene, and Soil-Transmitted Helminth Infection: A Systematic Review and Meta-Analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef] [Green Version]
- Speich, B.; Croll, D.; Fürst, T.; Utzinger, J.; Keiser, J. Effect of sanitation and water treatment on intestinal protozoa infection: A systematic review and meta-analysis. Lancet. Infect. Dis. 2016, 16, 87–99. [Google Scholar] [CrossRef]
- Nery, S.V.; Traub, R.J.; McCarthy, J.S.; Clarke, N.E.; Amaral, S.; Llewellyn, S.; Weking, E.; Richardson, A.; Campbell, S.J.; Gray, D.J.; et al. WASH for WORMS: A Cluster-Randomized Controlled Trial of the Impact of a Community Integrated Water, Sanitation, and Hygiene and Deworming Intervention on Soil-Transmitted Helminth Infections. Am. J. Trop. Med. Hyg. 2019, 100, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Bauza, V.; Guest, J. The effect of young children’s faeces disposal practices on child growth: Evidence from 34 countries. Trop. Med. Int. Health 2017, 22, 1233–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gera, T.; Shah, D.; Sachdev, H.S. Impact of Water, Sanitation and Hygiene Interventions on Growth, Non-diarrheal Morbidity and Mortality in Children Residing in Low- and Middle-income Countries: A Systematic Review. Indian Pediatrics 2018, 55, 381–393. [Google Scholar] [CrossRef]
- Freeman, M.C.; Garn, J.V.; Sclar, G.D.; Boisson, S.; Medlicott, K.; Alexander, K.T.; Penakalapatia, G.; Andersona, D.; Mahtani, A.G.; Grimes, J.E.T.; et al. The impact of sanitation on infectious disease and nutritional status: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 928–949. [Google Scholar] [CrossRef]
- Dickinson, K.L.; Patil, S.R.; Pattanayak, S.K.; Poulos, C.; Yang, J.-H. Nature’s Call: Impacts of sanitation choices in Orissa, India. Econ. Dev. Cult. Chang. 2015, 64, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Akobeng, A.K. Understanding systematic reviews and meta-analysis. Arch. Dis. Child. 2005, 90, 845–848. [Google Scholar] [CrossRef]
- Bhide, A.; Shah, P.S.; Acharya, G. A simplified guide to randomized controlled trials. Acta Obstet. Gynecol. Scand. 2018, 97, 380–387. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). WHO Guidelines on Sanitation and Health; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Paterson, C.; Baarts, C.; Launsø, L.; Verhoef, M.J. Evaluating complex health interventions: A critical analysis of the ‘outcomes’ concept. BMC Complement. Altern. Med. 2009, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaton, D.E.; Boers, M.; Tugwell, P. Assessment of health outcomes. In Textbook of Rheumatology, 9th ed.; Firesta, G.S., Budd, R.C., O’Dell, J.R., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2017; pp. 496–508. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moberg, J.; Kramer, M. A brief history of the cluster randomised trial design. J. R. Soc. Med. 2015, 108, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovićet, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Emerson, P.M.; Lindsay, S.W.; Alexander, N.; Bah, M.; Dibba, S.-M.; Faal, H.B.; Lowe, K.O.; McAdam, K.P.W.J.; Ratcliffe, A.A.; Walraven, G.E.L.; et al. Role of flies and provision of latrines in trachoma control: Cluster-randomised controlled trial. Lancet 2004, 363, 1093–1098. [Google Scholar] [CrossRef]
- Gebre, T.; Ayele, B.; Zerihun, M.; House, J.I.; Stoller, N.E.; Zhou, Z.; Ray, K.J.; Gaynor, B.D.; Porco, T.C.; Emerson, P.M.; et al. Latrine Promotion for Trachoma: Assessment of Mortality from a Cluster-Randomized Trial in Ethiopia. Am. J. Trop. Med. Hyg. 2011, 85, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Stoller, N.E.; Gebre, T.; Ayele, B.; Zerihun, M.; Assefa, Y.; Habte, D.; Zhou, Z.; Porco, T.C.; Keenan, J.D.; House, J.I.; et al. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: A cluster-randomized trial. Int. Health 2011, 3, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.R.; Arnold, B.F.; Salvatore, A.; Briceño, B.; Ganguly, S.; Colford, J.M., Jr.; Gertler, P.J. The effect of India’s Total Sanitation Campaign on defecation behaviors and child health in rural Madhya Pradesh: A cluster-randomized controlled trial. PLoS Med. 2014, 11, e1001709. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.J.; Djebbari, H.; Lopez, C.; Coulibaly, M.; Alzua, M.L. Effect of a community-led sanitation intervention on child diarrhoea and child growth in rural Mali: A cluster-randomised controlled trial. Lancet Glob. Health 2015, 3, e701–e711. [Google Scholar] [CrossRef] [Green Version]
- Briceño, B.; Coville, A.; Gertler, P.; Martinez, S. Are there synergies from combining hygiene and sanitation promotion campaigns: Evidence from a large-scale cluster-randomized trial in rural Tanzania. PLoS ONE 2017, 12, e0186228. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Ercumen, A.; Benjamin-Chung, J.; Arnold, B.F.; Das, S.; Haque, R.; Ashraf, S.; Parvez, S.M.; Unicomb, L.; Rahman, M.; et al. Effects of Water, Sanitation, Handwashing, and Nutritional Interventions on Child Enteric Protozoan Infections in Rural Bangladesh: A Cluster-Randomized Controlled Trial. Clin. Infect. Dis. 2018, 67, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luby, S.P.; Rahman, M.; Arnold, B.F.; Unicomb, L.; Ashraf, S.; Winch, P.J.; Stewart, C.P.; Begum, F.; Hussain, F.; Benjamin-Chung, J.; et al. Effects of water quality, sanitation, hand washing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: A cluster randomised controlled trial. Lancet Glob. Health 2018, 6, e302–e315. [Google Scholar] [CrossRef] [Green Version]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a DoseEscalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.; Olivia, S.; Shah, M. Scaling up sanitation: Evidence from an RCT in Indonesia. J. Dev. Econ. 2019, 138, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ercumen, A.; Benjamin-Chung, J.; Arnold, B.F.; Lin, A.; Hubbard, A.E.; Stewart, C.; Rahman, Z.; Parvez, S.M.; Unicomb, L.; Rahman, M.; et al. Effects of water, sanitation, handwashing and nutritional interventions on soil-transmitted helminth infections in young children: A cluster-randomized controlled trial in rural Bangladesh. PLoS Neglected Trop. Dis. 2019, 13, e0007323. [Google Scholar] [CrossRef] [Green Version]
- Pickering, A.J.; Njenga, S.M.; Steinbaum, L.; Swarthout, J.; Lin, A.; Arnold, B.F.; Stewart, C.P.; Dentz, H.N.; Mureithi, M.; Chieng, B.; et al. Effects of single and integrated water, sanitation, handwashing, and nutrition interventions on child soil-transmitted helminth and Giardia infections: A cluster-randomized controlled trial in rural Kenya. PLoS Med. 2019, 16, e1002841. [Google Scholar] [CrossRef] [Green Version]
- Steinbaum, L.; Mboya, J.; Mahoney, R.; Njenga, S.M.; Null, C.; Pickering, A.J. Effect of a sanitation intervention on soil-transmitted helminth prevalence and concentration in household soil: A cluster-randomized controlled trial and risk factor analysis. PLoS Neglected Trop. Dis. 2019, 13, e0007180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobban, F.; Postlethwaite, A.; Glentworth, D.; Pinfold, V.; Wainwright, L.; Dunn, G.; Clancy, A.; Haddock, G. A systematic review of randomised controlled trials of interventions reporting outcomes for relatives of people with psychosis. Clin. Psychol. Rev. 2013, 33, 372–382. [Google Scholar] [CrossRef]
- Safari, J.; Mohamed, H.; Dimoso, P.; Akyoo, W.; Odhiambo, F.; Mpete, R.; Massa, K.; Mwakitalima, A. Lessons learned from the national sanitation campaign in Njombe district, Tanzania. J. Water Sanit. Hyg. Dev. 2019, 9, 754–764. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Hayashi, M.A.L.; Eisenberg, J.N.S.; Brown, J. The Critical Role of Compliance in Delivering Health Gains from Environmental Health Interventions. Am. J. Trop. Med. Hyg. 2019, 100, 777–779. [Google Scholar] [CrossRef] [Green Version]
- Garn, J.V.; Sclar, G.D.; Freeman, M.C.; Penakalapati, G.; Alexander, K.T.; Brooks, P.; Rehfuess, E.A.; Boisson, S.; Medlicott, K.O.; Clasen, T.F. The impact of sanitation interventions on latrine coverage and latrine use: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Massa, K.; Kilamile, F.; Safari, E.; Seleman, A.; Mwakitalima, A.; Balengayabo, J.G.; Kassile, T.; Mangesho, P.E.; Mubyazi, G.M. Contributing to the debate on categorising shared sanitation facilities as ‘unimproved’: An account based on field researchers’ observations and householders’ opinions in three regions, Tanzania. PLoS ONE 2017, 12, e0185875. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, W.-P. Seven trials, seven question marks. Lancet Glob. Health 2015, 3, e659–e660. [Google Scholar] [CrossRef] [Green Version]
- Martin, N.A.; Hulland, K.R.S.; Dreibelbis, R.; Sultana, F.; Winch, P. Sustained adoption of water, sanitation and hygiene interventions: Systematic review. Trop. Med. Int. Health 2017, 23, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Herbert, R.D.; Kasza, J.; Bø, K. Analysis of randomised trials with long-term follow-up. BMC Med. Res. Methodol. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Nunan, D.; Heneghan, C.; Spencer, E.A. Catalogue of bias: Allocation bias. BMJ Evidence-Based Med. 2018, 23, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Rabiu, M.; Alhassan, M.B.; Ejere, H.O.; Evans, J.R. Environmental sanitary interventions for preventing active trachoma. Cochrane Database Syst. Rev. 2012, 2, CD004003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contzen, N.; De Pasquale, S.; Mosler, H.-J. Over-Reporting in Handwashing Self-Reports: Potential Explanatory Factors and Alternative Measurements. PLoS ONE 2015, 10, e0136445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Chu, H.; Murad, M.H.; Hong, C.; Qu, Z.; Cole, S.R.; Chen, Y. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.T.; Hum, R.J.; Lou, W.; Cheng, Y.-L. Effects of neighbourhood and household sanitation conditions on diarrhea morbidity: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0173808. [Google Scholar] [CrossRef] [Green Version]
- Bero, L.; Chartres, N.; Diong, J.; Fabbri, A.; Ghersi, D.; Lam, J.; Lau, A.; McDonald, S.; Mintzes, B.; Sutton, P.; et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: Concerns arising from application to observational studies of exposures. Syst. Rev. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Gu, G.; Zhou, Y.; Zhang, Y.; Cui, W. Increased prevalence of anxiety and depression symptoms in patients with coronary artery disease before and after percutaneous coronary intervention treatment. BMC Psychiatry 2016, 16, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopewell, S.; McDonald, S.; Clarke, M.J.; Egger, M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst. Rev. 2007, MR000010. [Google Scholar] [CrossRef] [PubMed]


| Database | Search String | Applied Filters |
|---|---|---|
| Cochrane Library-Trials | Advanced search: (WASH sanitation randomized controlled trial) | 2000–2019 English |
| MEDLINE Ovid | Advanced search: ((((effect OR impact) AND (sanitation OR WASH) AND interventions AND health AND outcomes) OR (disease OR diarrhea OR child growth)) AND (randomized AND controlled AND trial) AND (low AND middle AND income AND country)) | 2000–2019 Article Full text journals |
| PubMed | Advanced search: (sanitation interventions health outcomes randomized controlled trials) | Full text 2000–2019 Randomized controlled trial |
| Science Direct | Advanced search: (effect sanitation interventions health outcomes randomized controlled trials low- and middle-income countries) | Research article 2000–2019 |
| SCOPUS | Advanced search: (effect OR impact AND sanitation OR WASH AND interventions AND health AND outcomes OR diarrhea OR child AND growth AND randomized AND controlled AND trial AND low- AND middle- AND income AND country) | 2000–2019 Article English |
| Web of Science | Advanced search: TS = (effect AND sanitation AND interventions AND diarrhea AND child AND growth AND randomized AND controlled AND trials) | 2000–2019 English |
| Reference | Country/Continent of Trial/Trial Registration | % Access to Basic Sanitation | Sanitation Intervention Group | Intervention Duration (Years) | Sanitation Technology Option(s) | Sanitation Demand | Exposure Pathway(s) Based on the Study | Intervention Subsidy | Reasons for Loss to Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Emerson et al. [26] | Gambia (Africa) - | 3.5 | 2230 participants in 7 clusters | 2 | Non-ventilated pit latrine | Not specified | Vector Contact | Government subsidized | travelled, death, declined |
| Gebre et al. [27] | Ethiopia (Africa) NCT00322972 | - | 14,189 persons in 12 Subkebeles | 2.16 | Pit latrine with concrete slab | Not specified | Vector Contact | Government subsidized | - |
| Stoller et al. [28] | Ethiopia (Africa) NCT00322972 | - | 14,289 people in 12 Subkebeles | 2 | Simple pit latrine | Not specified | Vector | Material subsidy | - |
| Clasen et al. [3] | India (Asia) NCT01214785 | 9 (any type) | 10,014 individuals, including 1919 chn <5 in; 50 villages | 3.58 | Pour flush | Not specified | Water, Contact, food | Government subsidized | Death, absent, family dropout |
| Patil et al. [29] | India (Asia) NCT01465204 | 13.64 | 1683 chn < 5976 households in 40 villages | Not clear | Various | Not specified | Water, food contact | Government subsidized for national TSC | - |
| Dickinson et al. [17] | India (Asia) - | 25 (owned) | 1050 households, 1256 chn <5, 40 villages | 0.42 | Several under CLTS | CLTS triggering | Water | Government subsidized | - |
| Pickering et al. [30] | Mali (Africa) NCT01900912 | 22 (control) | 2365 households, 3508 chn <5, 60 villages | Not clear | Several under CLTS | CLTS triggering | Water | - | - |
| Briceño et al. [31] | Tanzania (Africa) NCT01465204 | 49.7 | 86 villages in 44 wards | 2.3 | Several under CLTS | CLTS triggering | Water, food contact | - | - |
| Lin et al. [32] | Bangladesh (Asia) NCT01590095 | 53 (owned) | 696 compounds in 90 clusters | 1 | Double pit Latrine with water seal | Not specified | Water, contact | Material subsidy | Moved, death, withdrew, no live birth, absent |
| Luby et al. [33] | Bangladesh (Asia) NCC01590095 | 54 (owned) | 696 compounds in 90 clusters | 1 | Double pit Latrine with water seal | Not specified | Water, contact food | Material subsidy | Moved, no live Birth, absent, refused, |
| Null et al. [34] | Kenya (Africa) NCT01704105 | 16 | 892 households 77 clusters | 1.5 | ‘Improved latrines’ | Not specified | Water, food contact | Material Subsidy, | Absent, died refused, no live birth, |
| Cameron et al. [35] | Indonesia (Asia) - | - | 80 villages | - | Several CLTS campaign | CLTS triggering | Contact, food water | - | - |
| Ercumen et al. [36] | Bangladesh (Asia) NCT01590095 | 53.4 (owned) | 696 women in 90 clusters, 1030 children | 1 | Concrete-lined double pit latrine (seal) | Not specified | Contact water | Provision of upgraded latrines | Moved, death, absent, no live birth, withdrew |
| Pickering et al. [37] | Kenya (Africa) NCT01704105 | 15.7 | 892 households in 77 clusters | 1.5 | Not specified | Not specified | Water, food contact | New latrines and upgrading existing ones | Absent, death, refused, no live birth |
| Stainbaum et al. [38] | Kenya (Africa) NCT01704105 | 15.7 | 892 households 77 clusters | 1.5 | ‘Improved latrines’ | Not specified | Water, food contact | Material subsidy | Absent, no live Birth, refused, death |
| Reference | Time When Post-Intervention Follow-up Was Done (In Years) | Enrolment Criteria | Intervention Adherence (%) | Health Outcome | Study Limitations | Key Findings | |||
| Emerson et al. [26] | 0.5 | Clusters randomly recruited in sets of three | First 0.5 years: 98% | Disease | Study done in low prevalence area. Fly catching without release induces catcher bias (unblinded) | Access to basic sanitation reduced fly eye contact Insignificant reduction in prevalence of trachoma in sanitation intervention | |||
| Gebre et al. [27] | 2.16 | Subkebekes randomly selected | 61.5 | Disease | No masking, insufficient sample size, no hygiene education | No effect of latrine construction on mortality (under 5 year old children). | |||
| Stoller et al. [28] | 1 and 2 | Subkebekes Randomly selected | 67.2 | Disease, Parasite | Flies not only transmission route, sanitation control varies in space and time, | Latrine construction offered no protection to prevalence of trachoma | |||
| Clasen et al. [3] | 1.5 | Household with child <4 years or pregnant woman | 36 | Disease, Growth, Parasite | Short follow-up period 1.5 year Self- and care-giver reported bias | No reduced exposure, prevention to diarrhea and STHs or child effect on malnutrition. | |||
| Patil et al. [29] | 1.75 | Villages randomly selected | 59 | Disease, Growth, Parasite | Short-term follow-up, contamination in the control group and self-reported outcomes | Increased coverage (19%), reduced open defecation 10%) but no improvements on diseases and child growth | |||
| Dickinson et al. [17] | 0.42 | Household with child <5 years | - | Disease, Growth | Study under-powered to statistically detect precise effects on diarrhea, | No statistically precise reductions in diarrhea, but increased anthropometric measurements of children <5 years of age | |||
| Pickering et al. [30] | 1.5 | Household with at least a child <10 years old | - | Disease, Growth | Self-reported measure, one follow-up in dry season, no universal access | No reduced diarrhea prevalence, increased child growth (<2) reduced open defecation and stunting (<5). Future work: Sanitation and height | |||
| Briceño et al. [31] | 1 | Households with a child <5 | - | Disease, Growth | No pre-intervention baseline characteristics, small changes in intermediate outcomes due to isolated interventions | Increased access (49.7–64.8%), reduced open defecation but the final effects of sanitation on child health were absent | |||
| Lin et al. [32] | 2.5 | Pregnant women, Chn ages 18–27 months | 85–87 | Parasite | Giardia genotype not determined, unknown protozoan infection status after intervention initiation but determined before 2 years. | Sanitation intervention reduced Childhood Giardia infections (9%) | |||
| Luby et al. [33] | 1 and 2 | Pregnant women, Index chn | ‘high’ | Disease, Growth | Caregiver-reported primary outcomes. Intervention in one socio-ecological zone in times of low prevalence of diarrhea | Sanitation intervention had no effect on child linear growth at year 2 but reduced the diarrhea prevalence (3.5%) than in the control (5.7%). | |||
| Null et al. [34] | 1 and 2 | Pregnant women, other requirements | >70: year 1, <25: year 2 | Disease, Growth | No observable indicators of actual behavior, compound and not community-level, focus on human feces not animal | Sanitation had no effect on diarrhea prevalence and child growth. | |||
| Cameron et al. [35] | 2 | Household with child <5 years | ‘low’ | Parasite, Growth | Partial compliance to treatment as 13.8% of the control was exposed to treatment | Associated decrease in roundworm infestations but no improvements in child growth. | |||
| Ercumen et al. [36] | 2.5 | Pregnant women in 1st or 2nd trimester, Index chn | 54 | Parasite | Poor instrumental sensitivity, only relative statistical power to detect relatively large effects, short follow-up for A. lumbricoides | Sanitation reduced T. trichiura (29%), had borderline effects on hookworm and no effect on A. lumbricoides. | |||
| Pickering et al. [37] | 2 | Village with at least 6 pregnant women Index chn | Year 1: 89–90 Year 2: 79–82 | Parasite | Imperfect uptake of targeted behaviour, limited power to detect effects on rareparasite infections | Sanitation had no effect on prevalence of Ascaris infection, and no reduction in Giardia | |||
| Steinbaum et al. [38] | 2 | Village with pregnant women, Index chn | Year 1: 89–90 Year 2: 79–82 | Parasite | No precise measures of compound defecation practices Soil analysis method only optimized for Ascaris, not Trichris or hookworm eggs | Sanitation had no effect on presence of eggs of total STH, Ascaris or Trichuris | |||
| Reference | Presence of Disease | Parasite Infestation | Child Growth | Main Indicator (s) for the Outcome | Total Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhoea | Trachoma | Protozoan | Helminthic | Other | Anthropometric | Prevalence | Mortality | Height | Weight | Other | ||
| Emerson et al. [26] | ✓ | ✓ | 1 | |||||||||
| Gebre et al. [27] | ✓ | ✓ | ✓ | 2 | ||||||||
| Stoller et al. [28] | ✓ | ✓ | ✓ | 2 | ||||||||
| Clasen et al. [3] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 3 | ||||
| Patil et al. [29] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 3 | ||||
| Dickinson et al. [17] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 2 | |||||
| Pickering et al. [30] | ✓ | ✓ | ✓ | ✓ | ✓ | 2 | ||||||
| Briceño et al. [31] | ✓ | ✓ | ✓ | ✓ | ✓ | 2 | ||||||
| Lin A et al. [32] | ✓ | ✓ | 1 | |||||||||
| Luby et al. [33] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 2 | |||||
| Null et al. [34] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 2 | |||||
| Cameron et al. [35] | ✓ | ✓ | ✓ | ✓ | ✓ | 2 | ||||||
| Ercumen et al. [36] | ✓ | ✓ | 1 | |||||||||
| Pickering et al. [37] Steinbaum et al. [38] | ✓ | ✓ ✓ | ✓ ✓ | 1 1 | ||||||||
| Health Outcome | Significant Effect of Sanitation Shown by Randomised Controlled Trial | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| Disease | Active trachoma | ✕ | ✕ | ✕ | ||||||||||||
| Reported diarrhoea | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✕ | |||||||||
| Parasite infection | protozoa | ✓ | ✕ | |||||||||||||
| Enteric helminths | ✕ | ✓ | ✓ | ✕ | ✕ | |||||||||||
| Other | ✕ | ✕ | ||||||||||||||
| Child growth (anthropometric) | Weight | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | |||||||
| Height | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ||||||||
| Other measure | ✕ | ✕ | ✓ | ✕ | ✕ | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, A.; Ncube, E.J.; Voyi, K. Effect of Sanitation Interventions on Health Outcomes: A Systematic Review of Cluster-Randomized Controlled Trials in Rural Communities of Low- and Middle-Income Countries. Int. J. Environ. Res. Public Health 2021, 18, 8313. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18168313
Kanda A, Ncube EJ, Voyi K. Effect of Sanitation Interventions on Health Outcomes: A Systematic Review of Cluster-Randomized Controlled Trials in Rural Communities of Low- and Middle-Income Countries. International Journal of Environmental Research and Public Health. 2021; 18(16):8313. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18168313
Chicago/Turabian StyleKanda, Artwell, Esper Jacobeth Ncube, and Kuku Voyi. 2021. "Effect of Sanitation Interventions on Health Outcomes: A Systematic Review of Cluster-Randomized Controlled Trials in Rural Communities of Low- and Middle-Income Countries" International Journal of Environmental Research and Public Health 18, no. 16: 8313. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18168313