The Management of the Cotyledonoid Leiomyoma of the Uterus: A Narrative Review of the Literature
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Preoperative Assessment
3.2. Surgical and Hormone Therapies
3.3. Differential Diagnosis
3.4. Pathological Findings
3.5. Histological Variants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, L.M.; Reed, R.J.; Sternberg, W.H. Cotyledonoid Dissecting Leiomyoma of the Uterus. the Sternberg Tumor. Am. J. Surg. Pathol. 1996, 20, 1455–1461. [Google Scholar] [CrossRef]
- Smith, C.C.; Gold, M.A.; Wile, G.; Fadare, O. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Review of Clinical, Pathological, and Radiological Features. Int. J. Surg. Pathol. 2012, 20, 330–341. [Google Scholar] [CrossRef]
- Buonomo, F.; Bussolaro, S.; Giorda, G.; Romano, F.; Biffi, S.; Ricci, G. Cotyledonoid Leiomyoma Clinical Characteristics, Imaging Features, and Review of the Literature. J. Ultrasound Med. 2020, 40, 1459–1469. [Google Scholar] [CrossRef]
- Preda, L.; Rizzo, S.; Gorone, M.S.; Fasani, R.; Maggioni, A.; Bellomi, M. MRI Features of Cotyledonoid Dissecting Leiomyoma of the Uterus. Tumori J. 2009, 95, 532–534. [Google Scholar] [CrossRef]
- David, M.P.; Homonnai, T.Z.; Deligdish, L.; Loewenthal, M. Grape-Like Leiomyomas of the Uterus. Int. Surg. 1975, 60, 238–239. [Google Scholar]
- Brand, A.H.; Scurry, J.P.; Planner, R.S.; Grant, P.T. Grapelike Leiomyoma of the Uterus. Am. J. Obstet. Gynecol. 1995, 173, 959–961. [Google Scholar] [CrossRef]
- Menolascino-Bratta, F.; Garcia de Barriola, V.; Naranjo de Gomez, M.; Garcia Tamayo, J.; Suarez, J.A.; Hernandez Chacon, A.V. Cotyledonoid Dissecting Leiomyoma (Sternberg Tumor): An Unusual Form of Leiomyoma. Pathol. Res. Pract. 1999, 195, 435–438; discussion 439. [Google Scholar] [CrossRef]
- Roth, L.M.; Reed, R.J. Cotyledonoid Leiomyoma of the Uterus: Report of a Case. Int. J. Gynecol. Pathol. 2000, 19, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, W.; Chan, J.K.; Liu, J.Y. Cotyledonoid Leiomyoma: A Benign Uterine Tumor with Alarming Gross Appearance. Arch. Pathol. Lab. Med. 2002, 126, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.B.; Al-Nafussi, A.; Beattie, G. Cotyledonoid Hydropic Intravenous Leiomyomatosis: A New Variant Leiomyoma. Histopathology 2002, 40, 245–252. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, Y.K.; Cho, J.H. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Case Report and Review of the Literature. J. Korean Med. Sci. 2002, 17, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Stewart, K.A.; Ireland-Jenkin, K.; Quinn, M.; Armes, J.E. Cotyledonoid Dissecting Leiomyoma. Pathology 2003, 35, 177–179. [Google Scholar]
- Gurbuz, A.; Karateke, A.; Kabaca, C.; Arik, H.; Bilgic, R. A Case of Cotyledonoid Leiomyoma and Review of the Literature. Int. J. Gynecol. Cancer 2005, 15, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.S.; Hanaa, B.; Faisal, A.S.; Najla, A.M. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Case Report of a Benign Uterine Tumor with Sarcomalike Gross Appearance and Review of Literature. Int. J. Gynecol. Pathol. 2006, 25, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Maimoon, S.; Wilkinson, A.; Mahore, S.; Bothale, K.; Patrikar, A. Cotyledonoid Leiomyoma of the Uterus. Indian J. Pathol. Microbiol. 2006, 49, 289–291. [Google Scholar] [PubMed]
- Mathew, M.; Gowri, V.; Al Hamdani, A.; Machado, L.; Rao, K.; Shabnam, S. Cotyledonoid Leiomyoma in Pregnancy. Obstet. Gynecol. 2007, 109, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Shelekhova, K.V.; Kazakov, D.V.; Michal, M. Cotyledonoid Dissecting Leiomyoma of the Uterus with Intravascular Growth: Report of Two Cases. Virchows Arch. 2007, 450, 119–121. [Google Scholar] [CrossRef]
- Weissferdt, A.; Maheshwari, M.B.; Downey, G.P.; Rollason, T.P.; Ganesan, R. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Case Report. Diagn. Pathol. 2007, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özçimen, E.E.; Kıyıcı, H.; Uçkuyu, A.; Akyürek, C. Cotyledonoid Dissecting Type Leiomyoma of the Uterus: A Case Report and Review of the Literature. Gynecol. Obstet. Reprod. Med. 2008, 14, 205–207. [Google Scholar]
- Misir, A.; Daya, D.; Sur, M. Cotyledonoid Dissecting Leiomyoma of the Uterus (Sternberg Tumour): A Clinicopathological Study of Six Cases. Can. J. Pathol. 2009, 1, 9–15. [Google Scholar]
- Adedipe, T.O.; Vine, S.J. Dissecting Cotyledonoid Leiomyoma: A Rare Cause of Chronic Intractable Menorrhagia (Not Amenable to Medical Treatment). Case Report. Eur. J. Gynaecol. Oncol. 2010, 31, 230–232. [Google Scholar] [PubMed]
- Agarwal, R.; Radhika, A.; Malik, R.; Radhakrishnan, G. Cotyledonoid Leiomyoma and Non-Descent Vaginal Hysterectomy. Arch. Gynecol. Obstet. 2010, 281, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Suzuki, K.; Hiruta, N. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Report of Four Cases. Am. J. Surg. Pathol. 2010, 118, 331–333. [Google Scholar] [CrossRef]
- Aggarwal, S.; Arora, V.K. An Unusual Variant of Leiomyoma Masquerading Peroperatively as Sarcoma. Indian J. Cancer 2011, 48, 120–121. [Google Scholar]
- Soleymani Majd, H.; Ismail, L.; Desai, S.A.; Reginald, P.W. Epithelioid Cotyledonoid Dissecting Leiomyoma: A Case Report and Review of the Literature. Arch. Gynecol. Obstet. 2011, 283, 771–774. [Google Scholar] [CrossRef]
- Gezginc, K.; Yazici, F.; Selimoglu, R.; Tavli, L. Cotyledonoid Dissecting Leiomyoma of the Uterus with Intravascular Growth in Postmenopausal Woman: A Case Presentation. Int. J. Clin. Oncol. 2011, 16, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, S.; Turgutalp, H.; Mungan, S.; Guvendi, G.; Guven, S. Cotyledonoid Leiomyoma of Uterus: A Case Report. Turk. J. Pathol. 2011, 27, 257–260. [Google Scholar] [CrossRef]
- Kim, N.R.; Park, C.Y.; Cho, H.Y. Cotyledonoid Dissecting Leiomyoma of the Uterus with Intravascular Luminal Growth: A Case Study. Korean J. Pathol. 2013, 47, 477–480. [Google Scholar] [CrossRef]
- Sellami, R.; Nasfi, A.; Doghri, R.; Nesrine, M.; Sassi, S.; Charfi, L.; Mrad, K.; Romdhane, K.B. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Report of Four Cases. J. Gynecol. Surg. 2013, 29, 23–26. [Google Scholar] [CrossRef]
- Bothale, A.A.; Bothale, K.A.; Mahore, S.D.; Wilkinson, A.R. Case Report: Cotyledonoid Leiomyoma. J. Basic Clin. Reprod. Sci. 2013, 2, 63–65. [Google Scholar] [CrossRef]
- Roth, L.M.; Kirker, J.A.; Insull, M.; Whittaker, J. Recurrent Cotyledonoid Dissecting Leiomyoma of the Uterus. Int. J. Gynecol. Pathol. 2013, 32, 215–220. [Google Scholar] [CrossRef]
- Makharoblidze, E.; Goishvili, N.; Mchedlishvili, M.; Khakhutaishvili, I.; Jangavadze, M. Unusual Types of Smooth Muscle Tumors of Uterine Corpus: Case Reports and Literature Review. Georgian Med. News 2013, 216, 7–11. [Google Scholar]
- Onu, D.O.; Fiorentino, L.M.; Bunting, M.W. Cotyledonoid Dissecting Leiomyoma as a Possible Cause of Chronic Lower Back Pain. BMJ Case Rep. 2013, 2013, bcr2013201350. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Toriyabe, K.; Senda, T.; Sakakura, Y.; Yoshida, K.; Asakura, T.; Taniguchi, H.; Nagao, K. Cotyledonoid Dissecting Leiomyoma Treated by Laparoscopic Surgery: A Case Report. Asian J. Endosc. Surg. 2013, 6, 122–125. [Google Scholar] [CrossRef]
- Chawla, I.; Bhardwaj, M.; Sareen, N.; Khattar, N. Epithelioid Cotyledonoid Leiomyoma of Uterus. BMJ Case Rep. 2014, 2014, bcr2013202434. [Google Scholar] [CrossRef] [Green Version]
- Meena, L.N.; Aggarwal, A.; Jain, S. Cotyledonoid Leiomyoma of Uterus. J. Obstet. Gynaecol. India 2014, 64, 146–147. [Google Scholar] [CrossRef] [Green Version]
- Geynisman, J.; Pagan, C.; Pirog, E.; Holcomb, K. Cotyledonoid Dissecting Leiomyoma. Int. J. Gynecol. Obstet. 2014, 125, 284. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.A.; Cheng, G.; Post, M.D.; Guntupalli, S. Cotyledonoid Dissecting Leiomyoma with Adipocytic Differentiation: A Case Report. Gynecol. Oncol. Rep. 2014, 11, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bas, S.; Selcuk, I.; Sirvan, L.; Yalcin, H.; Gungor, T. Cotyledonoid Myoma: A Distinct Entity and a Diagnostic Dilemma in Gynecology. J. Cases Obstet. Gynecol. 2015, 2, 91–93. [Google Scholar]
- Saeki, H.; Suzuki, C.; Yamasaki, S.; Hashizume, A.; Izumi, H.; Suzuki, F.; Ishi, K.; Nojima, M.; Hino, O. Cotyledonoid Dissecting Leiomyoma of the Uterus: Report of Two Cases. Arch. Gynecol. Obstet. 2015, 291, 357–361. [Google Scholar] [CrossRef]
- Motoshima, S.; Irie, H.; Nakazono, T.; Yamasaki, F.; Nakao, Y. Mri Findings of Cotyledonoid Dissecting Leiomyoma of the Uterus. Pak. J. Radiol. 2016, 21, 134–138. [Google Scholar]
- Raga, F.; Cholvi, S.; Pascual, C.; Boigues, D.; Sanchez, C.; Cano, A. More to be Learned about Cotyledonoid Dissecting Leiomyoma. Ultrasound Int. Open 2016, 2, E73–E74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, A.; Tanaka, H.; Iwasaki, S.; Wakui, Y.; Ikeda, H.; Suzuki, A. An Unusual Case of Uterine Cotyledonoid Dissecting Leiomyoma with Adenomyosis. Diagn. Pathol. 2016, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Wu, S.; Yang, R.; Zhao, L.; Sui, M.; Cui, M.; Chang, W. Cotyledonoid Dissecting Leiomyoma of the Uterus: A Report of Four Cases and a Review of the Literature. Oncol. Lett. 2016, 11, 2865–2868. [Google Scholar] [CrossRef]
- Buckshee, K.; Harsh, R.; Deepshikha, A.; Tanya, R.B. A Bizarre Highly Vascular Tumor with Alarming Presentation: A Diagnostic Dilemma. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 5622. [Google Scholar] [CrossRef] [Green Version]
- Merchant, K.; Chern, B.; Chew, S.H. Cotyledonoid Dissecting Leiomyoma with Intravascular Growth Pattern and Intra-Tumoural Endometrial Glands and Stroma: A Case Report. Case Report. Clin. Pathol. 2017, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sonmez, F.C.; Tosuner, Z.; Karasu, A.F.G.; Arici, D.S.; Dansuk, R. Cotyledonoid Dissecting Leiomyoma with Symplastic Features: Case Report. Rev. Bras. Ginecol. Obstet. 2017, 39, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Rahman, H.; Dubey, S.; Chavan, P.; Sherpa, M.L.; Sharma, B.K.; Khalda, E. Perinodular Hydropic Degeneration in a Case of Leiomyoma Uterus: A Rare Case Report and Review of Literature. Indian Obstet. Gynaecol. 2018, 8, 45–48. [Google Scholar]
- Smith, H.; Jung, N.; Carter, A.; Watson, M.; Singh, A. Post-Hysterectomy Extrauterine Cotyledonoid Leiomyoma in a 42-Year-Old Female. Urol. Case Rep. 2018, 19, 16–17. [Google Scholar] [CrossRef]
- Khatun, S.F.; Nazneen, T.; Khatun, S. A 48 Year Old Postmenopausal Woman with Dull Aching Lower Abdominal Pain and Heaviness in the Abdomen. Bangabandhu Sheikh Mujib Med. Univ. J. 2018, 11, 241–245. [Google Scholar] [CrossRef]
- Rocha, A.C.; Oliveira, M.; Luis, P.; Nogueira, M. Cotyledonoid Dissecting Leiomyoma of the Uterus: An Unexpected Diagnosis after Delivery. Acta Med. Port. 2018, 31, 223–227. [Google Scholar] [CrossRef]
- Tuli, A.G.; Goyal, S. Cotyledonoid Dissecting Leiomyoma of the Uterus. J. Datta Meghe Inst. Med. Sci. Univ. 2018, 13, 111. [Google Scholar] [CrossRef]
- Jamal, I.; Gupta, R.K.; Sinha, R.K.; Bhadani, P.P. Cotyledonoid Dissecting Leiomyoma: An Uncommon Form of a Common Disease. Obstet. Gynecol. Sci. 2019, 62, 362–366. [Google Scholar] [CrossRef]
- Kashima, J.; Tonooka, A.; Taguchi, A.; Funata, N.; Yasugi, T.; Hishima, T. A Cotyledonoid Dissecting Leiomyoma with an Intravascular Component and Adenomyosis Accompanied with Possible Multiple Lung Metastases: A Case Report. Hum. Pathol. Case Rep. 2019, 15, 79–82. [Google Scholar] [CrossRef]
- Ozdemir, O.; Sagır, G.; Akbas, B.; Guven, S.; Reis, A. A Case Report on Recurrent Cotyledonoid Dissecting Leiomyoma. J. Clin. Obstet. Gynecol. 2019, 29, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Lenz, J.; Chvátal, R.; Konečná, P. Dissecting Leiomyoma of the Uterus with Unusual Clinical and Pathological Features. Ceska Gynekol. 2020, 85, 197–200. [Google Scholar]
- Parker, W.H.; Turner, R.; Schwimer, S.; Foshag, L. Massive Cotyledenoid Leiomyoma Treated with Uterine-Conserving Surgery. F&S Rep. 2020, 1, 314–316. [Google Scholar]
- Niziurski, P.; Roszkowska-Purska, K.; Młodawski, J.; Młodawska, M.; Malmur, M. Cotyledonoid Dissecting Leiomyoma of the Uterus with Infiltration of Tumor Cells within Intestinal Wall: The First Polish Case Report of a Rare Uterine Tumor and Review of the Literature. Med. Stud. Stud. Med. 2020, 36, 328–331. [Google Scholar]
- Driss, M.; Zhioua, F.; Doghri, R.; Mrad, K.; Dhouib, R.; Romdhane, K.B. Cotyledonoid Dissecting Leiomyoma of the Uterus Associated with Endosalpingiosis. Arch. Gynecol. Obstet. 2009, 280, 1063–1065. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installe, A.J.; Guerriero, S.; et al. Terms, Definitions and Measurements to Describe Sonographic Features of Myometrium and Uterine Masses: A Consensus Opinion from the Morphological Uterus Sonographic Assessment (MUSA) Group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in Gynecological Disease (15): Clinical and Ultrasound Characteristics of Uterine Sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Togashi, K.; Koyama, T.; Yamaoka, T.; Fujiwara, T.; Fujii, S. Diffusely Enlarged Uterus: Evaluation with MR Imaging. Radiographics 2003, 23, 1423–1439. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Koyama, T.; Saga, T.; Morisawa, N.; Fujimoto, K.; Mikami, Y.; Togashi, K. The Utility of Diffusion-Weighted MR Imaging for Differentiating Uterine Sarcomas from Benign Leiomyomas. Eur. Radiol. 2008, 18, 723–730. [Google Scholar] [CrossRef]
- Rubisz, P.; Ciebiera, M.; Hirnle, L.; Zgliczyńska, M.; Łoziński, T.; Dzięgiel, P.; Kobierzycki, C. The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium. Int. J. Mol. Sci. 2019, 20, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, H.; Maruo, T.; Samoto, T. Increased Expression of Bcl-2 Protein in Human Uterine Leiomyoma and its Up-Regulation by Progesterone. J. Clin. Endocrinol. Metab. 1997, 82, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Pajak, E.; Liszka, L.; Pajak, J.; Golka, D. Immunohistochemical Profile of Cotyledonoid Dissecting Leiomyoma of the Uterus. Virchows Arch. 2010, 457, 216–217. [Google Scholar]

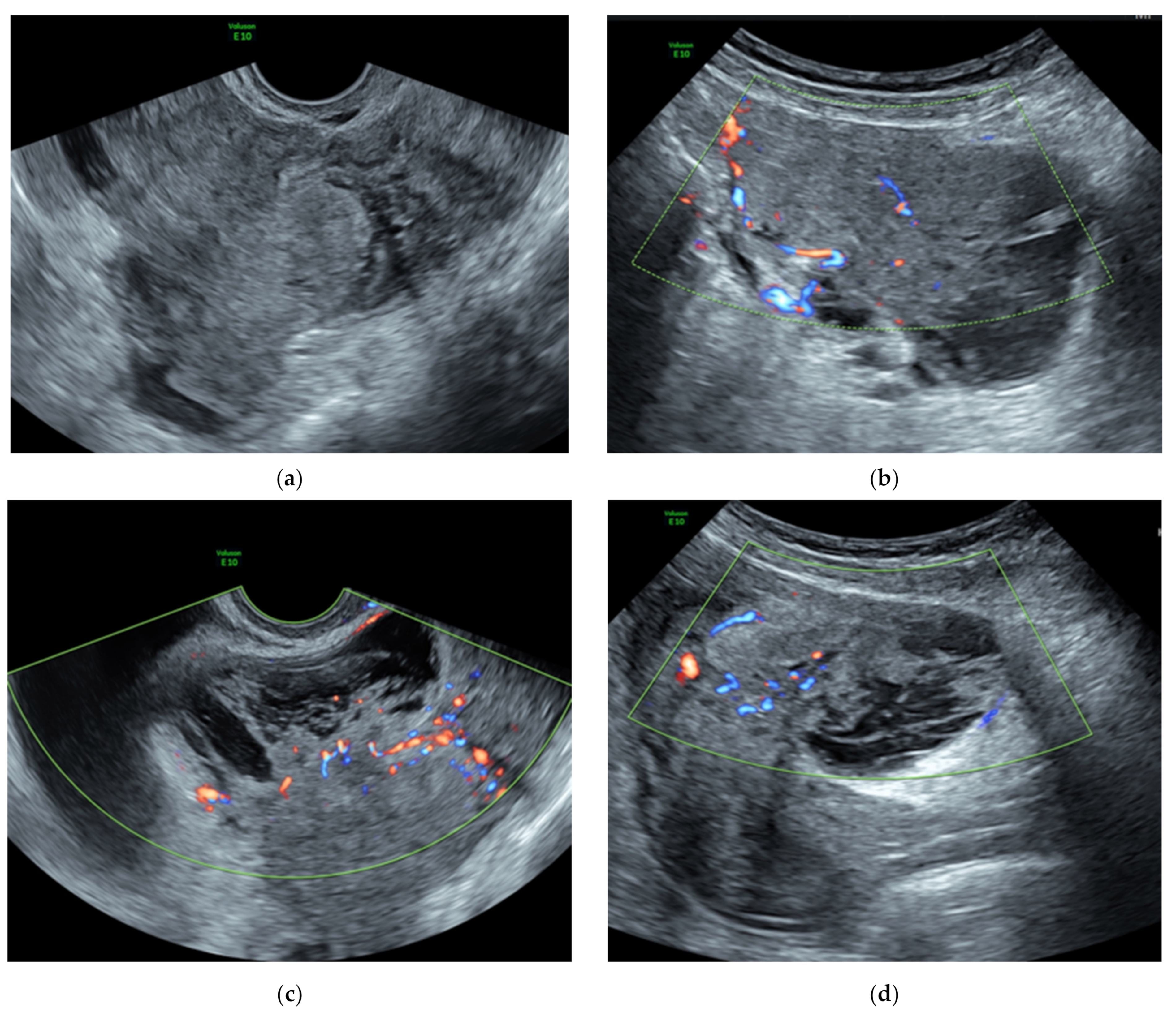
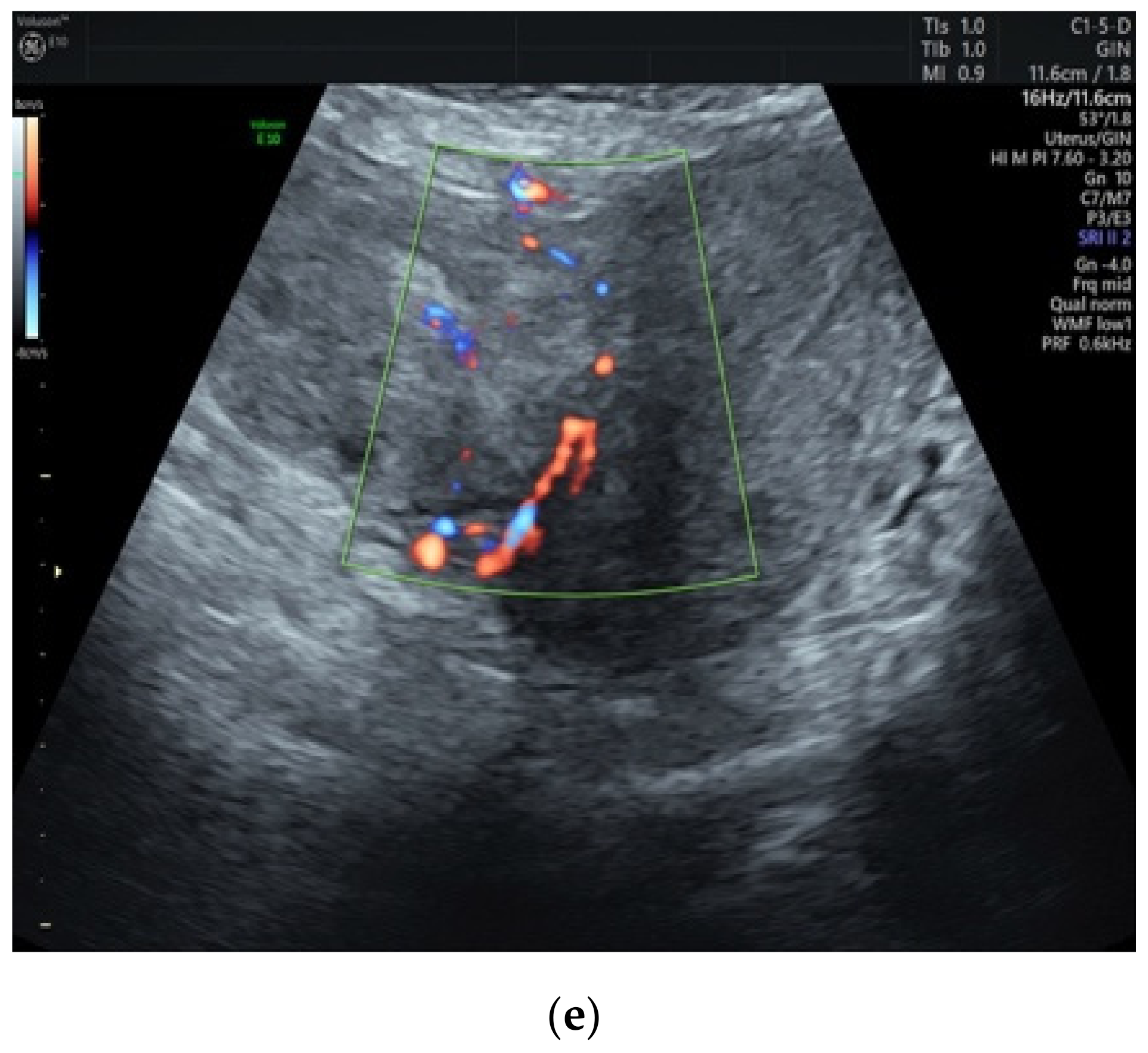
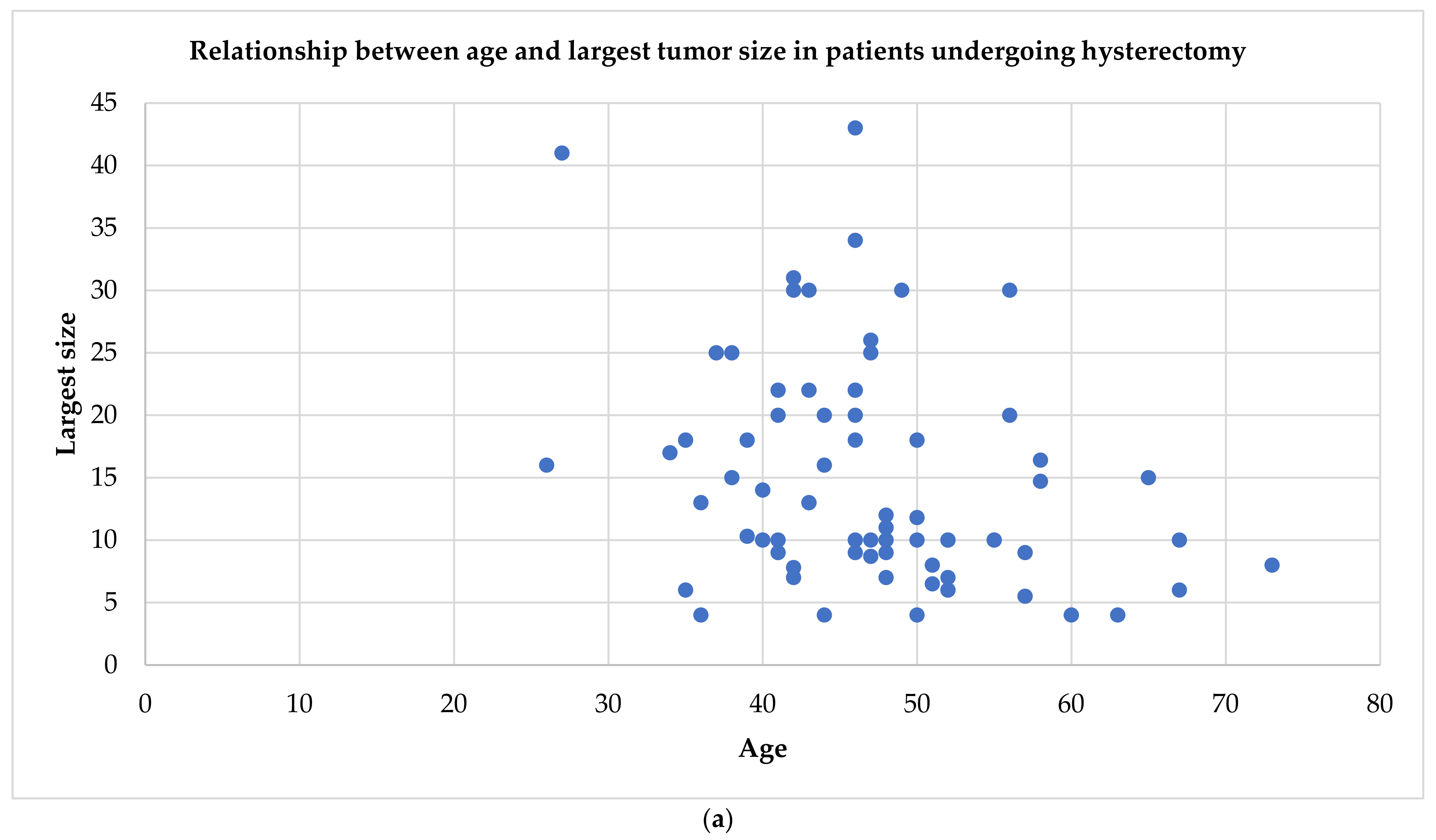

| First Author | Year of Publication | Number of Cases Described | Age | Clinical Symptoms | Tumor Largest Size (cm) * | Tumor Volume (cm3) | Pathologic Type | Therapy |
|---|---|---|---|---|---|---|---|---|
| David [5] | 1975 | 2 | 48; 65 | AUB, P, uterine prolapse | 12;15 | NS | 2 CDL | 2 TH |
| Brand [6] | 1995 | 1 | 24 | Abdominal swelling | NS | NS | CDL | M |
| Roth [1] | 1996 | 4 | 23–41 | Pelvic mass, AUB | 10–25 (25 cm) | NS | 4 CDL | 3 TH ± BSO, 1 M |
| Menolascino-Bratta [7] | 1999 | 1 | 26 | P | 16 | 2240 | CDL | TH + BSO + A |
| Roth [8] | 2000 | 1 | 46 | Menometrorrhagia | 34 | 11560 | CL | TH + BSO |
| Cheuk [9] | 2002 | 1 | 55 | Menorrhagia, uterine prolapse | 10 | 360 | CL | TH + BSO |
| Jordan [10] | 2002 | 6 | 34–46 | P, pelvic mass, menorrhagia | 10–22 (18 cm) | NS | 6 CDL (4 with intravenous leiomyomatosis) | 1 M, 5 subtotal/TH ± BSO |
| Kim [11] | 2002 | 1 | 26 | AUB | 12 | NS | CDL | M |
| Stewart [12] | 2003 | 1 | 58 | Pelvic mass | 16.4 | 1697.4 | CDL | TH + BSO+O |
| Gurbuz [13] | 2005 | 1 | 67 | Abdominal mass | 10 | 120 | CL | TH + BSO |
| Saeed [14] | 2006 | 1 | 27 | P, constipation | 41 | 13837.5 | CDL | TH + BSO |
| Maimoon [15] | 2006 | 1 | 40 | Urinary retention | 10 | NS | CL with hydropic degeneration | TH + SO |
| Mathew [16] | 2007 | 1 | 30 | P in pregnancy | 30 | NS | CL with hydropic degeneration | M |
| Shelekhova [17] | 2007 | 2 | 73;48 | Pelvic mass, P | 8;9 | NS, 260 | 2 CDL (1 with intravascular growth, 1 with hydropic degeneration) | 2 TH + BSO |
| Weissferdt [18] | 2007 | 1 | 52 | Menorrhagia, P | 6 | 72 | CDL with hydropic degeneration | TH + S |
| Özcimen [19] | 2008 | 1 | 38 | AUB, P | 15 | 750 | CDL | TH + BS |
| Misir [20] | 2009 | 6 | 35–57 | Pelvic mass, abdominal swelling, irregular menses, menorrhagia | 5–30 | NS | 6 CDL (2 epithelioid, 5 with hydropic degeneration) | 4 TH, 1 TH + BSO + O + A, 1 TH + SO |
| Preda [4] | 2009 | 1 | 41 | Incidental | 9 | NS | CDL | TH + ovariectomy |
| Adelipe [21] | 2010 | 1 | 37 | Menorrhagia | NS | NS | CDL | TH + O |
| Agarwal [22] | 2010 | 1 | 52 | Polymenorrhagia | 10 | NS | CDL | TH |
| Fukunaga [23] | 2010 | 4a | 35–56 | Constipation, abdominal mass, hypermenorrhea, P | 4–30 | NS | 4 CDLb | 4 TH ± BSO |
| Aggarwal [24] | 2011 | 1 | 52 | Menorrhagia, uterine prolapse | 10 | NS | CL with hydropic degeneration | TH + SO |
| Soleymani [25] | 2011 | 1 | 63 | AUB, P | 4 | 36 | Epithelioid CDL | TH + BSO |
| Gezginig [26] | 2011 | 1 | 57 | P | 9 | 162 | CDL with intravascular growth | TH + BSO |
| Ersöz [27] | 2011 | 1 | 51 | Menorrhagia | 8 | NS | CL | TH + BSO |
| Kim [28] | 2013 | 1 | 43 | Pelvic mass and P | 13 | NS | CL | TH + BSO |
| Sellami [29] | 2013 | 4c | 47–52 | Abdominal mass, hyper-menorrhea | 7–30 (10 cm) | NS | 4 CDLd | 1 M, 3 subtotal/TH ± BSO |
| Bothale [30] | 2013 | 1 | 39 | Pelvic mass | 18 | 4320 | CL | TH |
| Roth [31] | 2013 | 1 | 21 | Menorrhagia | 6.5 | 143 | CDL | M, then TH + BSO |
| Makharoblidze [32] | 2013 | 1 | 42 | Menorrhagia, polymenorrhea, P | 31 | 27900 | CDL | TH |
| Onu [33] | 2013 | 1 | 50 | Chronic lower back pain | 4 | 30 | CDL | RH + BSO + O + A |
| Tanaka [34] | 2013 | 1 | 36 | Incidental | 10 | NS | CDL | M |
| Chawla [35] | 2014 | 1 | 42 | Dysmenorrhea, P | 7 | 245 | Epithelioid CL | TH + BSO |
| Meena [36] | 2014 | 1 | 40 | P | 14 | NS | CDL | TH + BSO |
| Geynisman [37] | 2014 | 1 | 50 | P | 18 | 1890 | CDL | TH + BSO |
| Blake [38] | 2014 | 1 | 56 | AUB | 20 | NS | CDL with adipocytic differentia-tion | RH + BSO + O |
| Bas [39] | 2015 | 1 | 46 | P, menometrorrhagia | 22 | 5280 | CL | TH |
| Saeki [40] | 2015 | 2 | 44; 31 | Incidental, P | 20;13 | NS, M **: 497.3 | 2 CDL | 1 TH + BSO, 1 GnRHanalogs + M |
| Motoshima [41] | 2016 | 1 | 39 | Abdominal mass | 14 | 1176 | CDL with hydropic degeneration | M |
| Raga [42] | 2016 | 1 | 28 | Menorrhagia, dysmenorrhea | 15 | 1435.4 | CDL | UPA + M |
| Shimizu [43] | 2016 | 1 | 40 | Menorrhagia | 10 | 810 | CDL | TH + BS |
| Xu [44] | 2016 | 4 | 37–55 | Pelvic mass | 7–30 | NS, 12000, 5250, NS | 4 CDL | 4 TH ± BSO |
| Buckshee [45] | 2017 | 1 | 29 | P | 26 | 6760 | CDL | M |
| Merchant [46] | 2017 | 1 | 35 | Dysmenorrhea and menorrhagia | 10 | 210 | CDL with intra-vascular growth | M |
| Sonmez [47] | 2017 | 1 | 38 | P | 13.5 | 1275.8 | CL with symplastic features | M |
| Rahman [48] | 2018 | 1 | 46 | P, abdominal swelling | 43 | 31648 | CDL with hydropic degeneration | TH + SO |
| Smith [49] | 2018 | 1 | 42 | Pelvic vaginal cuff mass | 8 | 240 | CDL | Resection |
| Khatun [50] | 2018 | 1 | 48 | P | 10 | 320 | CDL | TH + BSO |
| Rocha [51] | 2018 | 1 | 38 | Menorrhagia, P | 25 | 13800 | CDL of the uterus and the ovary | TH + BSO |
| Tuli [52] | 2018 | 1 | 50 | Menorrhagia | 11.8 | 681.5 | CDL with hydropic degeneration | TH + BSO |
| Jamal [53] | 2019 | 1 | 60 | P | 4 | NS | CDL | TH |
| Kashima [54] | 2019 | 1 | 43 | Menorrhagia | 22 | 1694 | CDL with intravascular growth | TH + BSO |
| Özdemir [55] | 2019 | 1 | 34 | Menorrhagia, P | 17 | NS | CDL | TH |
| Lenz [56] | 2020 | 1 | 64 | P | NS | NS | CDL | TH + R |
| Parker [57] | 2020 | 1 | 39 | Abdominal mass | 28 | NS | CL | M |
| Niziurski [58] | 2020 | 1 | 41 | AUB, pelvic mass | 20 | 5100 | CDL with hydropic degeneration | TH + BSO + intestine resection |
| Buonomo [3] | 2020 | 13 | 30–67 | Pelvic mass, P, incidental, metrorrhagia | 4–14,7 | 489.6, 280, 132.8, 305, 770, 480, 210, 49, 165, 1411 M **: 157.5, 106.5, 1188 | 3 CDL, 10 CL | 3 M, 4 TH + BSO, 2 TH + BS, 1 TH + S, 2 RH + BSO, 1 RH + S + O |
| Pelvic Ultrasound | Magnetic Resonance Imaging | During Surgery |
|---|---|---|
|
|
|
| Gross Pathology | Microscopic Elements |
|---|---|
|
|
| Positivity [9,20] | Negativity [25] |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonomo, F.; Bussolaro, S.; Fiorillo, C.d.A.; Giorda, G.; Romano, F.; Biffi, S.; Ricci, G. The Management of the Cotyledonoid Leiomyoma of the Uterus: A Narrative Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 8521. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18168521
Buonomo F, Bussolaro S, Fiorillo CdA, Giorda G, Romano F, Biffi S, Ricci G. The Management of the Cotyledonoid Leiomyoma of the Uterus: A Narrative Review of the Literature. International Journal of Environmental Research and Public Health. 2021; 18(16):8521. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18168521
Chicago/Turabian StyleBuonomo, Francesca, Sofia Bussolaro, Clarice de Almeida Fiorillo, Giorgio Giorda, Federico Romano, Stefania Biffi, and Giuseppe Ricci. 2021. "The Management of the Cotyledonoid Leiomyoma of the Uterus: A Narrative Review of the Literature" International Journal of Environmental Research and Public Health 18, no. 16: 8521. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18168521







