Effects of Breastfeeding on Endometriosis-Related Pain: A Prospective Observational Study
Abstract
:1. Introduction
- Exclusive breastfeeding: the infant only receives breast milk without any additional food or drink, not even water.
- Predominant breastfeeding: maternal milk, maternal milk squeezed or donated, plus non-nutritional liquids such as water, infusions, and sugary drinks.
- Complementary feeding: maternal milk and any other drink or food added, including non-human milk.
- Artificial feeding: no maternal milk feeding, only other sources of drinks and food, including non-human milk.
2. Materials and Methods
3. Results
3.1. Exclusive Breastfeeding
3.2. Effect of Breastfeeding on Ovarian Endometriomas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Porpora, M.G.; Koninckx, P.R.; Piazze, J.; Natili, M.; Colagrande, S.; Cosmi, E.V. Correlation between endometriosis and pelvic pain. J. Am. Assoc. Gynecol. Laparosc. 1999, 6, 429–434. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–958. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M. Endometriosis and mechanisms of pelvic pain. J. Minim Invasive Gynecol. 2009, 16, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Porpora, M.G.; Vinci, V.; Bernardo, S.; Lodise, P.; Sollazzo, P.; Sergi, M.E.; Saldari, M.; Pace, G.; Vittori, G.; et al. Diffusion tensor imaging and tractography to evaluate sacral nerve root abnormalities in endometriosis-related pain: A pilot study. Eur. Radiol. 2014, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Vinci, V.; De Vito, C.; Migliara, G.; Anastasi, E.; Ticino, A.; Resta, S.; Catalano, C.; Benedetti Panici, P.; Manganaro, L. The role of magnetic resonance imaging-diffusion tensor imaging in predicting pain related to endometriosis: A preliminary study. J. Minim Invasive Gynecol. 2018, 25, 661–669. [Google Scholar] [CrossRef]
- Porpora, M.G.; Tomao, F.; Manganaro, L.; Yazdanian, D.; Fuggetta, E.; Piccioni, M.G.; Benedetti Panici, P.; Benagiano, G. Impaired uterine artery flow associated with the presence of ovarian endometrioma: Preliminary results of a prospective study. J. Ovarian Res. 2014, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Masciullo, L.; Viscardi, M.F.; Piacenti, I.; Scaramuzzino, S.; Cavalli, A.; Piccioni, M.G.; Porpora, M.G. A deep insight into pelvic pain and endometriosis: A review of the literature from pathophysiology to clinical expressions. Minerva Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Kitawaki, J.; Kado, N.; Ishihara, H.; Koshiba, H.; Kitaoka, Y.; Honjo, H. Endometriosis: The pathophysiology as an estrogen-dependent disease. J. Steroid Biochem. Mol. Biol. 2002, 83, 149–155. [Google Scholar] [CrossRef]
- Chen, H.; Malentacchi, F.; Fambrini, M.; Harrath, A.H.; Huang, H.; Petraglia, F. Epigenetics of estrogen and progesterone receptors in endometriosis. Reprod. Sci. 2020, 11, 1967–1974. [Google Scholar] [CrossRef]
- Zeitoun, K.; Takayama, K.; Sasano, H.; Suzuki, T.; Moghrabi, N.; Andersson, S.; Johns, A.; Meng, L.; Putman, M.; Carr, B.; et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: Failure to metabolize 17beta-estradiol. J. Clin. Endocrinol. Metab. 1998, 83, 4474–4480. [Google Scholar] [PubMed] [Green Version]
- Esfandiari, F.; Heidari Khoei, H.; Saber, M.; Favaedi, R.; Piryaei, A.; Moini, A.; Shahhoseini, M.; Ramezanali, F.; Ghaffari, F.; Baharvand, H. Epithelial-to-mesenchymal transition contributes to the downregulation of progesterone receptor expression in endometriosis lesions. J. Steroid. Biochem. Mol. Biol. 2021, 212, 105943, published online ahead of print. [Google Scholar]
- Ma, L.; Andrieu, T.; McKinnon, B.; Duempelmann, L.; Peng, R.W.; Wotzkow, C.; Müller, C.; Mueller, M.D. Epithelial-to-mesenchymal transition contributes to the downregulation of progesterone receptor expression in endometriosis lesions. J. Steroid Biochem. Mol. Biol. 2021, 212, 105943. [Google Scholar] [CrossRef] [PubMed]
- Battin, D.A.; Marrs, R.P.; Fleiss, P.M.; Mishell, D.R., Jr. Effect of suckling on serum prolactin, luteinizing hormone, follicle-stimulating hormone, and estradiol during prolonged lactation. Obstet. Gynecol. 1985, 65, 785–788. [Google Scholar] [PubMed]
- Piacenti, I.; Viscardi, M.F.; Masciullo, L.; Sangiuliano, C.; Scaramuzzino, S.; Piccioni, M.G.; Muzii, L.; Benedetti Panici, P.; Porpora, M.G. Dienogest versus continuous oral levonorgestrel/EE in patients with endometriosis: What’s the best choice? Gynecol. Endocrinol. 2021, 2, 1–5. [Google Scholar]
- Porpora, M.G.; Pultrone, D.C.; Bellavia, M.; Franco, C.; Crobu, M.; Cosmi, E.V. Reproductive outcome after laparoscopic treatment of endometriosis. Clin. Exp. Obstet. Gynecol. 2002, 29, 271–273. [Google Scholar]
- Horton, J.; Sterrenburg, M.; Lane, S.; Maheshwari, A.; Li, T.C.; Cheong, Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 592–632. [Google Scholar] [CrossRef] [PubMed]
- Zullo, F.; Spagnolo, E.; Saccone, G.; Acunzo, M.; Xodo, S.; Ceccaroni, M.; Berghella, V. Endometriosis and obstetrics complications: A systematic review and meta-analysis. Fertil. Steril. 2017, 108, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Porpora, M.G.; Tomao, F.; Ticino, A.; Piacenti, I.; Scaramuzzino, S.; Simonetti, S.; Imperiale, L.; Sangiuliano, C.; Masciullo, L.; Manganaro, L.; et al. Endometriosis and Pregnancy: A Single Institution Experience. Int. J. Environ. Res. Public Health 2020, 7, 401. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.; Lalani, S.; Xie, R.H.; Shen, M.; Singh, S.S.; Wen, S.W. Association between surgically diagnosed endometriosis and adverse pregnancy outcomes. Fertil. Steril. 2018, 109, 142–147. [Google Scholar]
- Matsuzaki, S.; Nagase, Y.; Ueda, Y.; Lee, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Endo, M.; et al. The association of endometriosis with placenta previa and postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, L.; Reschini, M.; La Vecchia, I.; Candotti, G.; Somigliana, E.; Vercellini, P. Endometriosis and spontaneous hemoperitoneum in pregnancy: Evaluation of the magnitude of the risk in women becoming pregnant via in vitro fertilization. Fertil. Steril. 2021, 115, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.A.; Negrini, R.; Da Silva Ferreira, R.D. Premature birth in women with endometriosis: A systematic review and meta-analysis. Reprod. Sci. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.; De Padova, M.; Falagario, M.; D’Alterio, M.N.; Di Spiezio Sardo, A.; Alonso Pacheco, L.; Carugno, J.T.; Nappi, L. Endometriosis and adverse pregnancy outcome. Minerva Obstet. Gynecol. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [Green Version]
- Nußbaumer-Streit, B.; Gartlehner, G. WHO-Leitlinie: Beratung von Müttern zur Verbesserung von Stillpraktiken [WHO Guideline: Counselling of women to improve breastfeeding practices]. Gesundheitswesen 2020, 82, 274–279. [Google Scholar] [PubMed]
- Vekemans, M. Postpartum contraception: The lactational amenorrhea method. Eur. J. Contracept. Reprod. Health Care 1997, 2, 105–111. [Google Scholar] [CrossRef]
- Moore, E.R.; Bergman, N.; Anderson, G.C.; Medley, N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst. Rev. 2016, 25, 11. [Google Scholar] [CrossRef] [Green Version]
- Hortu, I.; Ozceltik, G.; Karadadas, E.; Erbas, O.; Yigitturk, G.; Ulukus, M. The Role of Ankaferd Blood Stopper and Oxytocin as Potential Therapeutic Agents in Endometriosis: A Rat Model. Curr. Med. Sci. 2020, 40, 556–562. [Google Scholar] [CrossRef]
- Karimi, F.Z.; Miri, H.H.; Khadivzadeh, T.; Maleki-Saghooni, N. The effect of mother-infant skin-to-skin contact immediately after birth on exclusive breastfeeding: A systematic review and meta-analysis. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 46–56. [Google Scholar] [CrossRef]
- Lau, Y.; Tha, P.H.; Ho-Lim, S.; Wong, L.Y.; Lim, P.I.; Citra Nurfarah, B.; Shorey, S. An analysis of the effects of intrapartum factors, neonatal characteristics, and skin-to-skin contact on early breastfeeding initiation. Matern. Child Nutr. 2018, 14, 12492. [Google Scholar] [CrossRef] [Green Version]
- Farland, L.V.; Prescott, J.; Sasamoto, N.; Tobias, D.K.; Gaskins, A.J.; Stuart, J.J.; Carusi, D.A.; Chavarro, J.E.; Horne, A.W.; Rich-Edwards, J.W.; et al. Endometriosis and risk of adverse pregnancy outcomes. Obstet. Gynecol. 2019, 134, 527–536. [Google Scholar] [CrossRef]
- Miura, M.; Ushida, T.; Imai, K.; Wang, J.; Moriyama, Y.; Nakano-Kobayashi, T.; Osuka, S.; Kikkawa, F.; Kotani, T. Adverse effects of endometriosis on pregnancy: A case-control study. BMC Pregnancy Childbirth 2019, 19, 373. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, J.; Yan, S.; Wu, H.; Bai, T. Early feeding behaviors and breastfeeding outcomes after cesarean section. Breastfeed. Med. 2019, 14, 325–333. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.; Xue, M.; Zhou, Y.; Sun, P.; Leng, J. Not having been breastfed may protect Chinese women from developing deep infiltrating endometriosis: Results from subgroup analyses of the FEELING study. Reprod. Sci. 2019, 8, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef] [Green Version]
- Farland, L.V.; Eliassen, A.H.; Tamimi, R.M.; Spiegelman, D.; Michels, K.B.; Missmer, S.A. History of breast feeding and risk of incident endometriosis: Prospective cohort study. BMJ 2017, 358, 3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benagiano, G.; Brosens, I. In utero exposure and endometriosis. J. Matern. Fetal. Neonatal. Med. 2014, 3, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, H.; Zhang, Q.; Hua, K. Influence of early-life factors on the development of endometriosis. Eur. J. Contracept. Reprod. Health Care 2019, 24, 216–221. [Google Scholar] [CrossRef]
- Alex, A.; Bhandary, E.; McGuire, K.P. Anatomy and physiology of the breast during pregnancy and lactation. Adv. Exp. Med. Biol. 2020, 1252, 3–7. [Google Scholar] [PubMed]
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [CrossRef]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Malspeis, S.; Willett, W.C.; Hunter, D.J. Reproductive history and endometriosis among premenopausal women. Obstet. Gynecol. 2004, 104, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.A.; Kim, M.Y.; Lee, S.R.; Jeong, K.A.; Chung, H.W. Factors related to dysmenorrhea among Vietnamese and Vietnamese marriage immigrant women in South Korea. Obstet. Gynecol. Sci. 2013, 56, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porpora, M.G.; Pallante, D.; Ferro, A.; Crisafi, B.; Bellati, F.; Benedetti Panici, P. Pain and ovarian endometrioma recurrence after laparoscopic treatment of endometriosis: A long-term prospective study. Fertil. Steril. 2010, 93, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Chapron, C.; Fauconnier, A.; Dubuisson, J.B.; Barakat, H.; Vieira, M.; Bréart, G. Deep infiltrating endometriosis: Relation between severity of dysmenorrhoea and extent of disease. Hum. Reprod. 2003, 18, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Vercellini, P.; Trespidi, L.; De Giorgi, O.; Cortesi, I.; Parazzini, F.; Crosignani, P.G. Endometriosis and pelvic pain: Relation to disease stage and localization. Fertil. Steril. 1996, 65, 299–304. [Google Scholar] [CrossRef]
- Borghini, R.; Porpora, M.G.; Casale, R.; Marino, M.; Palmieri, E.; Greco, N.; Donato, G.; Picarelli, A. Irritable bowel syndrome-like disorders in endometriosis: Prevalence of nickel sensitivity and effects of a low-nickel diet. An Open-Label Pilot Study. Nutrients 2020, 12, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganò, D.; Zara, F.; Usai, P. Irritable bowel syndrome and endometriosis: New insights for old diseases. Dig. Liver. Dis. 2018, 50, 213–219. [Google Scholar] [CrossRef]
- Porpora, M.G.; Scaramuzzino, S.; Sangiuliano, C.; Piacenti, I.; Bonanni, V.; Piccioni, M.G.; Ostuni, R.; Masciullo, L.; Benedetti Panici, P. High prevalence of autoimmune diseases in women with endometriosis: A case-control study. Gynecol. Endocrinol. 2020, 36, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Karadadas, E.; Hortu, I.; Ak, H.; Ergenoglu, A.M.; Karadadas, N.; Aydin, H.H. Evaluation of complement system proteins C3a, C5a and C6 in patients of endometriosis. Clin. Biochem. 2020, 81, 15–19. [Google Scholar] [CrossRef]
- Chen, H.; Vannuccini, S.; Capezzuoli, T.; Ceccaroni, M.; Mubiao, L.; Shuting, H.; Wu, Y.; Huang, H.; Petraglia, F. Comorbidities and quality of life in women undergoing first surgery for endometriosis: Differences between chinese and italian population. Reprod. Sci. 2021, 28, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.G.; Borders, N.; Leeman, L.M.; Albers, L.L. Does spontaneous genital tract trauma impact postpartum sexual function? J. Midwifery Womens Health 2009, 54, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Âhlund, S.; Radestad, I.; Zwedberg, S.; Lindgren, H. Perineal Pain the first year after childbirth and uptake of postpartum check-up—A Swedish cohort study. Midwifery 2019, 78, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Barrett, G.; Pendry, E.; Peacock, J.; Victor, C.; Thakar, R.; Manyonda, I. Women’s sexual health after childbirth. BJOG 2000, 107, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Leeman, L.M.; Rogers, R.G. Sex after childbirth: Postpartum sexual function. Obstet. Gynecol. 2012, 119, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Harlow, B.L.; Chekos, A.K.; Repke, J.T. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am. J. Obstet. Gynecol. 2001, 184, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Takami, M.; Kajiyama, R.; Miyagi, E.; Aoki, S. Characteristics of ovarian endometriomas during pregnancy. J. Obstet. Gynaecol. Res. 2021, 47, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Sepulcri, R.P.; Do Amaral, V.F. Depressive symptoms, anxiety, and quality of life in women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 142, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Burgio, M.A.; Laganà, A.S.; Sicilia, A.; Prosperi Porta, R.; Porpora, M.G.; Ban Frangež, H.; Di Venti, G.; Triolo, O. Breastfeeding education: Where are we going? A systematic review article. Iran J. Public Health 2016, 45, 970–977. [Google Scholar]

| Age at delivery | mean ± SD |
| 33.8 ± 4.7 | |
| BMI | mean ± SD |
| 22.2 ± 2.7 | |
| Previous medical treatment | n (%) |
| 51 (41.4) | |
| NSAID intake | n (%) |
| 109 (88.6) | |
| Parity | n (%) |
| 0 | 84 (68.3) |
| 1 | 27 (21.9) |
| 2 | 12 (9.8) |
| Stage at previous surgery | n (%) |
| Stage III | 66 (53.6) |
| Stage IV | 57 (46.4) |
| Surgical findings | n (%) |
| Peritoneal lesions | 74 (60.1) |
| Adhesions | 62 (50.4) |
| DIE | 39 (31.7) |
| Unilateral endometriomas | 76 (61.8) |
| Bilateral endometriomas | 47 (38.2) |
| Ovarian endometriomas at the beginning of pregnancy | n (%) |
| Unilateral | 39 (76.4) |
| Bilateral | 12 (23.6) |
| Average size of ovarian endometriomas at pregnancy (cm) | mean ± SD |
| 3.25 ± 0.91 | |
| Prevalence of pain symptoms before pregnancy | n (%) |
| Dysmenorrhea | 101 (82.1) |
| Dyspareunia | 74 (60.1) |
| CPP | 91 (73.9) |
| Mean VAS score | mean ± SD |
| Dysmenorrhea | 6.1 ± 3.6 |
| Dyspareunia | 5.1 ± 2.8 |
| CPP | 4.8 ± 2 |
| Type of delivery | n (%) |
| Vaginal | 60 (48.8) |
| Cesarean section | 63 (51.2) |
| Gestational age at delivery | n (%) |
| Preterm (<37 weeks) | 18 (14.6) |
| Term (≥37 weeks) | 105 (85.4) |
| Months of postpartum amenorrhea | mean ± SD |
| 5.6 ± 4.3 |
| Dysmenorrhea | ||||||
| Group A (n = 17) | p-Value | Group B (n = 19) | p-Value | Group C (n = 33) | p-Value | |
| T0 | 6.9 | 4.7 | 6.5 | |||
| T3 * | 2 | 0.001 | 3.6 | 0.04 | 2.7 | 0.002 |
| T6 * | 4.6 | 0.004 | 3.2 | 0.002 | 2.1 | <0.001 |
| T12 | 5.4 | n.s. | 3.7 | n.s. | 2.4 | <0.001 |
| T24 | 5.4 | n.s. | 3.6 | n.s. | 4.3 | <0.001 |
| Dyspareunia | ||||||
| Group A (n = 9) | p-Value | Group B (n = 22) | p-Value | Group C (n = 15) | p-Value | |
| T0 | 5.4 | n.s. | 5.5 | n.s. | 5.4 | n.s. |
| T3 | 4.9 | n.s. | 5.2 | n.s. | 5.1 | n.s. |
| T6 | 4.8 | n.s. | 4.7 | n.s. | 4.6 | n.s. |
| T12 | 5.3 | n.s. | 4.9 | n.s. | 4.4 | n.s. |
| T24 | 5.2 | n.s. | 5.1 | n.s. | 4.7 | n.s. |
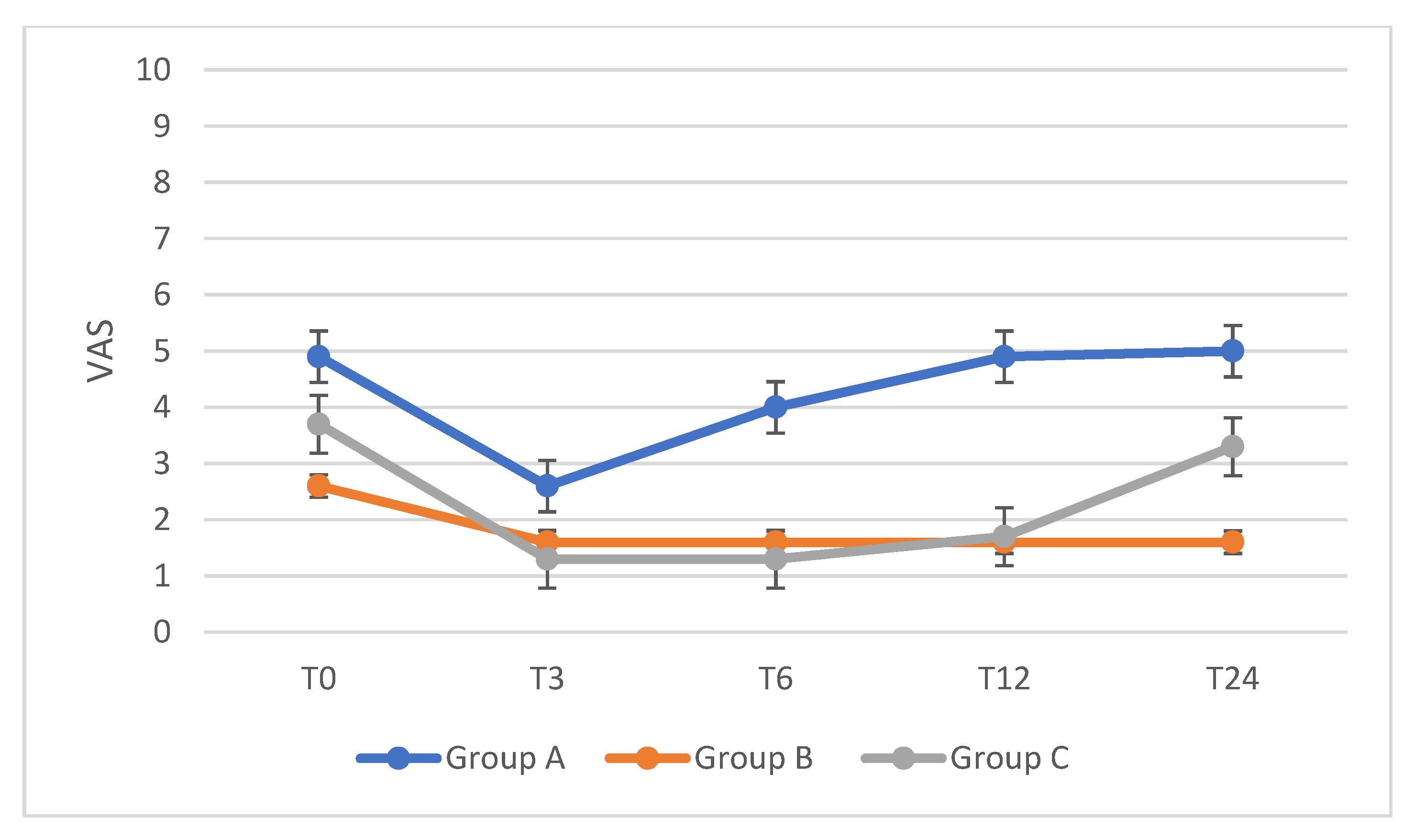
| CPP | ||||||
| Group A (n = 19) | p-Value | Group B (n = 25) | p-Value | Group C (n = 23) | p-Value | |
| T0 | 4.9 | 2.6 | 3.7 | |||
| T3 | 2.6 | 0.05 | 1.6 | n.s. | 1.3 | 0.002 |
| T6 | 4.0 | n.s. | 1.6 | n.s. | 1.3 | 0.002 |
| T12 | 4.9 | n.s. | 1.6 | n.s. | 1.7 | 0.04 |
| T24 | 5.0 | n.s. | 1.6 | n.s. | 3.3 | n.s. |
| All Patients (n = 51) (mean cm ± SD) | Exclusive Breastfeeding Patients (n = 42) (mean cm ± SD) | p-Value | |
|---|---|---|---|
| Before pregnancy | 3.25 ± 0.91 | 3.23 ± 0.91 | |
| 6-month follow-up | 3.01 ± 0.81 | 2.98 ± 0.79 | 0.041 |
| 24-month follow-up | 2.71 ± 0.79 | 2.69 ± 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi Porta, R.; Sangiuliano, C.; Cavalli, A.; Hirose Marques Pereira, L.C.; Masciullo, L.; Piacenti, I.; Scaramuzzino, S.; Viscardi, M.F.; Porpora, M.G. Effects of Breastfeeding on Endometriosis-Related Pain: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 10602. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182010602
Prosperi Porta R, Sangiuliano C, Cavalli A, Hirose Marques Pereira LC, Masciullo L, Piacenti I, Scaramuzzino S, Viscardi MF, Porpora MG. Effects of Breastfeeding on Endometriosis-Related Pain: A Prospective Observational Study. International Journal of Environmental Research and Public Health. 2021; 18(20):10602. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182010602
Chicago/Turabian StyleProsperi Porta, Romana, Chiara Sangiuliano, Alessandra Cavalli, Laila Cristine Hirose Marques Pereira, Luisa Masciullo, Ilaria Piacenti, Sara Scaramuzzino, Maria Federica Viscardi, and Maria Grazia Porpora. 2021. "Effects of Breastfeeding on Endometriosis-Related Pain: A Prospective Observational Study" International Journal of Environmental Research and Public Health 18, no. 20: 10602. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182010602







