Transwoman Elite Athletes: Their Extra Percentage Relative to Female Physiology
Abstract
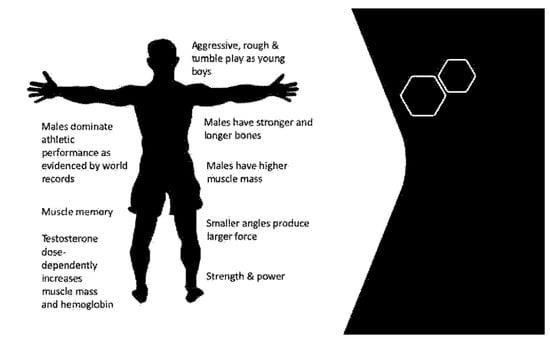
:1. Introduction
2. Male Physiology Provides an Athletic Performance Advantage
2.1. Prenatal Testosterone and the Male-like Brain
2.2. Testosterone and Muscle Mass
2.3. Testosterone and Bone Structure
2.4. Testosterone and the Cardiorespiratory System
3. Male Physiology Cannot Be Reformatted into Female Physiology by Estrogen Therapy
3.1. Difficulties in Achieving Female Levels of Circulating Testosterone in Estrogen-Treated Transwomen
3.2. Estrogen Therapy Does Not Reformat Male-Like Brain Networks
3.3. Estrogen Therapy Does Not Reformat Male Skeletal Architecture but Does Decrease Muscle Mass
3.4. Estrogen Therapy and Effects on the Cardiorespiratory System
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheuvront, S.N.; Carter, R.; DeRuisseau, K.C.; Moffatt, R.J. Running performance differences between men and women: An update. Sports Med. 2005, 35, 1017–1024. [Google Scholar] [CrossRef]
- Nevill, A.M.; Whyte, G. Are there limits to running world records? Med. Sci. Sports Exerc. 2005, 37, 1785–1788. [Google Scholar] [CrossRef]
- Seiler, S.; de Koning, J.J.; Foster, C. The fall and rise of the gender difference in elite anaerobic performance 1952–2006. Med. Sci. Sports Exerc. 2007, 39, 534–540. [Google Scholar] [CrossRef]
- Berthelot, G.; Thibault, V.; Tafflet, M.; Escolano, S.; El Helou, N.; Jouven, X.; Hermine, O.; Toussaint, J.-F. The citius end: World records progression announces the completion of a brief ultra-physiological quest. PLoS ONE 2008, 3, e1552. [Google Scholar] [CrossRef]
- Thibault, V.; Guillaume, M.; Berthelot, G.; El Helou, N.; Schaal, K.; Quinquis, L.; Nassif, H.; Tafflet, M.; Escolano, S.; Hermine, O.; et al. Women and Men in Sport Performance: The Gender Gap has not Evolved since 1983. J. Sports Sci. Med. 2010, 9, 214–223. [Google Scholar]
- Kennedy, C.L. A New Frontier for Women’s Sports (Beyond Title IX). Gend. Issues 2010, 27, 78–90. [Google Scholar] [CrossRef]
- Bretherton, I.; Thrower, E.; Grossmann, M.; Zajac, J.D.; Cheung, A.S. Cross-sex hormone therapy in Australia: The prescription patterns of clinicians experienced in adult transgender healthcare. Intern. Med. J. 2019, 49, 182–188. [Google Scholar] [CrossRef]
- Randolph, J.F., Jr. Gender-Affirming Hormone Therapy for Transgender Females. Clin. Obstet. Gynecol. 2018, 61, 705–721. [Google Scholar] [CrossRef]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef]
- McCarthy, M.M. Sex differences in the developing brain as a source of inherent risk. Dialogues. Clin. Neurosci. 2016, 18, 361–372. [Google Scholar] [CrossRef]
- de Vries, G.J.; Sodersten, P. Sex differences in the brain: The relation between structure and function. Horm. Behav. 2009, 55, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Isgor, C.; Sengelaub, D.R. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm. Behav. 1998, 34, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Isgor, C.; Sengelaub, D.R. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J. Neurobiol. 2003, 55, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, A.J.; Gozzi, A.; Bifone, A. Community structure and modularity in networks of correlated brain activity. Magn. Reson. Imaging 2008, 26, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef] [Green Version]
- Tunç, B.; Solmaz, B.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Calkins, M.E.; Ruparel, K.; Gur, R.E.; Gur, R.C.; Verma, R. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 20150111. [Google Scholar] [CrossRef] [Green Version]
- Robazza, C.; Bortoli, L. Perceived impact of anger and anxiety on sporting performance in rugby players. Psychol. Sport Exerc. 2007, 8, 875–896. [Google Scholar] [CrossRef]
- Bartlett, M.L.; Abrams, M.; Byrd, M.; Treankler, A.S.; Houston-Norton, R. Advancing the Assessment of Anger in Sports: Gender Differences and STAXI-2 Normative Data for College Athletes. J. Clin. Sport Psychol. 2018, 12, 114–128. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Hanin, Y. Perceived impact of anger on performance of skilled karate athletes. Psychol. Sport Exerc. 2011, 12, 242–249. [Google Scholar] [CrossRef]
- Abrams, M. Anger Management in Sport, 1st ed.; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- Bartlett, M.L.; Abrams, M. Anger and aggression in sport. In APA Handbooks in Psychology Series. APA Handbook of Sport and Exercise Psychology; Anshel, T.A.P.M.H., Steinfeldt, J.A., Eds.; American Psychological Association: Washington, DC, USA, 2019; pp. 509–528. [Google Scholar]
- Deaner, R.O. Physiology does not explain all sex differences in running performance. Med. Sci. Sports Exerc. 2013, 45, 146–147. [Google Scholar] [CrossRef] [Green Version]
- Deaner, R.O. Distance running as an ideal domain for showing a sex difference in competitiveness. Arch. Sex. Behav. 2013, 42, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Neave, N.; Morais, R.N.; Kilduff, L.; Taylor, S.R.; Butovskaya, M.; Fink, B.; Manning, J.T. Digit ratio (2D:4D), testosterone, cortisol, aggression, personality and hand-grip strength: Evidence for prenatal effects on strength. Early Hum. Dev. 2016, 100, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.B.; Kim, K.H. Why is digit ratio correlated to sports performance? J. Exerc. Rehabil. 2016, 12, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Bönte, W.; Procher, V.D.; Urbig, D.; Voracek, M. Digit Ratio (2D:4D) Predicts Self-Reported Measures of General Competitiveness, but Not Behavior in Economic Experiments. Front. Behav. Neurosci. 2017, 11, 238. [Google Scholar] [CrossRef] [Green Version]
- Mailhos, A.; Buunk, A.P.; del Arca, D.; Tutte, V. Soccer players awarded one or more red cards exhibit lower 2D:4D ratios. Aggress. Behav. 2016, 42, 417–426. [Google Scholar] [CrossRef]
- Voracek, M.; Reimer, B.; Dressler, S.G. Digit ratio (2D:4D) predicts sporting success among female fencers independent from physical, experience, and personality factors. Scand. J. Med. Sci. Sports 2010, 20, 853–860. [Google Scholar] [CrossRef]
- Healy, M.L.; Gibney, J.; Pentecost, C.; Wheeler, M.J.; Sonksen, P.H. Endocrine profiles in 693 elite athletes in the postcompetition setting. Clin. Endocrinol. 2014, 81, 294–305. [Google Scholar] [CrossRef]
- Sale, D.G. Neuromuscular function. In Gender Differences in Metabolism: Practical and Nutritional Implications; Tarnopolsky, M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; Chapter 4; pp. 61–86. [Google Scholar]
- Handelsman, D.J. Sex differences in athletic performance emerge coinciding with the onset of male puberty. Clin. Endocrinol. 2017, 87, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 1996, 335, 1–7. [Google Scholar] [CrossRef]
- Bhasin, S.; Woodhouse, L.J.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.; Magliano, L.; Dzekov, C.; et al. Testosterone dose-response relationships in healthy young men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1172–E1181. [Google Scholar] [CrossRef] [Green Version]
- Hirschberg, A.L. Hyperandrogenism in Female Athletes. J. Clin. Endocrinol. Metab. 2019, 104, 503–505. [Google Scholar] [CrossRef] [Green Version]
- Davidyan, A.; Pathak, S.; Baar, K.; Bodine, S.C. Maintenance of muscle mass in adult male mice is independent of testosterone. PLoS ONE 2021, 16, e0240278. [Google Scholar] [CrossRef]
- Hirschberg, A.L.; Knutsson, J.E.; Helge, T.; Godhe, M.; Ekblom, M.; Bermon, S.; Ekblom, B. Effects of moderately increased testosterone concentration on physical performance in young women: A double blind, randomised, placebo controlled study. Br. J. Sports Med. 2019, 54, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Riggs, B.L.; Spelsberg, T.C. Skeletal effects of estrogen. Endocr. Rev. 1994, 15, 275–300. [Google Scholar]
- Orwoll, E.S.; Klein, R.F. Osteoporosis in men. Endocr. Rev. 1995, 16, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Laubach, L.L. Comparative muscular strength of men and women: A review of the literature. Aviat. Space Environ. Med. 1976, 47, 534–542. [Google Scholar] [PubMed]
- Huseynov, A.; Zollikofer, C.P.; Coudyzer, W.; Gascho, D.; Kellenberger, C.; Hinzpeter, R.; Ponce de León, M.S. Developmental evidence for obstetric adaptation of the human female pelvis. Proc. Natl. Acad. Sci. USA 2016, 113, 5227–5232. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, M.A.; Wassersug, R.J.; Rosenberg, K.R. From transsexuals to transhumans in elite athletics—The implications of osteology (and other issues) in leveling the playing field. In Transgender Athletes in Competitive Sport; Routledge: London, UK, 2017; p. 238. [Google Scholar]
- Fornalski, S.; Gupta, R.; Lee, T.Q. Anatomy and biomechanics of the elbow joint. Tech. Hand Up. Extrem. Surg. 2003, 7, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Carey, M.A.; Card, J.W.; Voltz, J.W.; Arbes, S.J.; Germolec, D.R.; Korach, K.S.; Zeldin, D.C. It’s all about sex: Gender, lung development and lung disease. Trends Endocrinol. Metab. 2007, 18, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Thurlbeck, W.M. Postnatal human lung growth. Thorax 1982, 37, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellemare, F.; Jeanneret, A.; Couture, J. Sex differences in thoracic dimensions and configuration. Am. J. Respir. Crit. Care Med. 2003, 168, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Leinwand, L.A. Sex is a potent modifier of the cardiovascular system. J. Clin. Investig. 2003, 112, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. The sex difference in haemoglobin levels in adults-mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef]
- Coviello, A.D.; Kaplan, B.; Lakshman, K.M.; Chen, T.; Singh, A.B.; Bhasin, S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J. Clin. Endocrinol. Metab. 2008, 93, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, M.; Cheung, A.S.; Zajac, J.D. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 603–616. [Google Scholar] [CrossRef]
- Kenney, W.L.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise, 5th ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Gardner, I.H.; Safer, J.D. Progress on the road to better medical care for transgender patients. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 553–558. [Google Scholar] [CrossRef]
- Liang, J.; Jolly, D.; Chan, K.J.; Safer, J.D. Testosterone Levels Achieved by Medically Treated Transgender Women in a United States Endocrinology Clinic Cohort. Endocr. Pract. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- Leinung, M.C.; Feustel, P.; Joseph, J. Hormonal Treatment of Transgender Women with Oral Estradiol. Transgend. Health 2018, 3, 74–81. [Google Scholar] [CrossRef]
- SoRelle, J.A.; Jiao, R.; Gao, E.; Veazey, J.; Frame, I.; Quinn, A.M.; Day, P.; Pagels, P.; Gimpel, N.; Patel, K. Impact of Hormone Therapy on Laboratory Values in Transgender Patients. Clin. Chem. 2019, 65, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Jarin, J.; Pine-Twaddell, E.; Trotman, G.; Stevens, J.; Conard, L.A.; Tefera, E.; Gomez-Lobo, V. Cross-Sex Hormones and Metabolic Parameters in Adolescents with Gender Dysphoria. Pediatrics 2017, 139, e20163173. [Google Scholar] [CrossRef] [PubMed]
- Guillamon, A.; Junque, C.; Gil, E.G. A Review of the Status of Brain Structure Research in Transsexualism. Arch. Sex. Behav. 2016, 45, 1615–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreukels, B.P.; Guillamon, A. Neuroimaging studies in people with gender incongruence. Int. Rev. Psychiatry 2016, 28, 120–128. [Google Scholar] [CrossRef]
- Mueller, S.C.; Landré, L.; Wierckx, K.; T’Sjoen, G. A Structural Magnetic Resonance Imaging Study in Transgender Persons on Cross-Sex Hormone Therapy. Neuroendocrinology 2017, 105, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Seiger, R.; Hahn, A.; Hummer, A.; Kranz, G.S.; Ganger, S.; Woletz, M.; Kraus, C.; Sladky, R.; Kautzky, A.; Kasper, S.; et al. Subcortical gray matter changes in transgender subjects after long-term cross-sex hormone administration. Psychoneuroendocrinology 2016, 74, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zubiaurre-Elorza, L.; Junque, C.; Gómez-Gil, E.; Guillamon, A. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J. Sex. Med. 2014, 11, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.M.; Manzouri, A.H.; Savic, I. Structural connections in the brain in relation to gender identity and sexual orientation. Sci. Rep. 2017, 7, 17954. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.C.; Wierckx, K.; Jackson, K.; T’Sjoen, G. Circulating androgens correlate with resting-state MRI in transgender men. Psychoneuroendocrinology 2016, 73, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Kranz, G.; Hahn, A.; Kaufmann, U.; Tik, M.; Ganger, S.; Seiger, R.; Hummer, A.; Windischberger, C.; Kasper, S.; Lanzenberger, R. Effects of testosterone treatment on hypothalamic neuroplasticity in female-to-male transgender individuals. Brain Struct. Funct. 2018, 223, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Linn, M.C.; Petersen, A.C. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child. Dev. 1985, 56, 1479–1498. [Google Scholar] [CrossRef]
- Voyer, D.; Voyer, S.; Bryden, M.P. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychol. Bull. 1995, 117, 250–270. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Nowell, A. Sex differences in mental test scores, variability, and numbers of high-scoring individuals. Science 1995, 269, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Miles, C.; Green, R.; Hines, M. Estrogen treatment effects on cognition, memory and mood in male-to-female transsexuals. Horm. Behav. 2006, 50, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Georgakis, M.K.; Dimitriou, N.G.; Vichos, T.; Katsimpris, A.; Petridou, E.T.; Papadopoulos, F.C. Gender-affirming hormone treatment and cognitive function in transgender young athletes: A systematic review and meta-analysis. Psychoneuroendocrinology 2020, 119, 104721. [Google Scholar] [CrossRef] [PubMed]
- Van Goozen, S.H.; Cohen-Kettenis, P.T.; Gooren, L.J.; Frijda, N.H.; Van De Poll, N.E. Gender differences in behaviour: Activating effects of cross-sex hormones. Psychoneuroendocrinology 1995, 20, 343–363. [Google Scholar] [CrossRef]
- Pol, H.E.H.; Schnack, H.G.; Mandl, R.C.; Brans, R.G.; van Haren, N.E.; Baaré, W.F.; van Oel, C.; Collins, D.L.; Evans, A.C.; Kahn, R.S. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage 2006, 31, 482–488. [Google Scholar]
- Kim, T.-H.; Kim, G.-W.; Kim, S.-K.; Jeong, G.-W. Brain activation-based sexual orientation in female-to-male transsexuals. Int. J. Impot. Res. 2016, 28, 31–38. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.K.; Jeong, G.W. Cerebral gray matter volume variation in female-to-male transsexuals: A voxel-based morphometric study. Neuroreport 2015, 26, 1119–1125. [Google Scholar] [CrossRef]
- McCarthy, M.M. Multifaceted origins of sex differences in the brain. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 20150106. [Google Scholar] [CrossRef]
- Beking, T.; Geuze, R.; van Faassen, M.; Kema, I.; Kreukels, B.; Groothuis, T. Prenatal and pubertal testosterone affect brain lateralization. Psychoneuroendocrinology 2018, 88, 78–91. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Lew, J.; Albaugh, M.D.; Botteron, K.N.; Hudziak, J.J.; Fonov, V.S.; Collins, D.L.; Ducharme, S.; McCracken, J.T. Sex-specific associations of testosterone with prefrontal-hippocampal development and executive function. Psychoneuroendocrinology 2017, 76, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimshaw, G.; Sitarenios, G.; Finegan, J. Mental rotation at 7 years: Relations with prenatal testosterone levels and spatial play experiences. Brain Cogn. 1995, 29, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Elbers, J.M.H.; Asscheman, H.; Seidell, J.; Gooren, L.J.G. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am. J. Physiol. 1999, 276, E317–E325. [Google Scholar] [CrossRef]
- Wiik, A.; Lundberg, T.R.; Rullman, E.; Andersson, D.P.; Holmberg, M.; Mandić, M.; Brismar, T.B.; Leinhard, O.D.; Chanpen, S.; Flanagan, J.N.; et al. Muscle strength, size and composition following 12 months of gender-affirming treatment in transgender individuals: Retained advantage for the transwomen. bioRxiv 2019, 105, e805–e813. [Google Scholar]
- Roberts, T.A.; Smalley, J.; Ahrendt, D. Effect of gender affirming hormones on athletic performance in transwomen and transmen: Implications for sporting organisations and legislators. Br. J. Sports Med. 2020, 55, 577–583. [Google Scholar] [CrossRef]
- Harper, J. Race times for transgender athletes. J. Sport. Cult. Identities 2015, 6, 1–9. [Google Scholar] [CrossRef]
- van Kesteren, P.; Lips, P.; Gooren, L.J.; Asscheman, H.; Megens, J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin. Endocrinol. 1998, 48, 347–354. [Google Scholar] [CrossRef]
- Lee, H.; McGovern, K.; Finkelstein, J.S.; Smith, M.R. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer 2005, 104, 1633–1637. [Google Scholar] [CrossRef]
- Galvao, D.A.; Nosaka, K.; Taaffe, D.R.; Spry, N.; Kristjanson, L.J.; McGuigan, M.R.; Suzuki, K.; Yamaya, K.; Newton, R.U. Resistance training and reduction of treatment side effects in prostate cancer patients. Med. Sci. Sports Exerc. 2006, 38, 2045–2052. [Google Scholar] [CrossRef]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Malone, S.C.; Parliament, M.B.; Scott, C.G.; Venner, P.M.; Quinney, H.A.; Jones, L.W.; D’Angelo, M.E.S.; et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2003, 21, 1653–1659. [Google Scholar] [CrossRef]
- Fu, X.; Wang, H.; Hu, P. Stem cell activation in skeletal muscle regeneration. Cell Mol. Life Sci. 2015, 72, 1663–1677. [Google Scholar] [CrossRef] [Green Version]
- Bruusgaard, J.C.; Johansen, I.B.; Egner, I.M.; Rana, Z.A.; Gundersen, K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc. Natl. Acad. Sci. USA 2010, 107, 15111–15116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egner, I.M.; Bruusgaard, J.C.; Eftestøl, E.; Gundersen, K. A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. J. Physiol. 2013, 591, 6221–6230. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K. Excitation-transcription coupling in skeletal muscle: The molecular pathways of exercise. Biol. Rev. Camb. Philos. Soc. 2011, 86, 564–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundersen, K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J. Exp. Biol. 2016, 219 Pt 2, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Staron, R.S.; Leonardi, M.J.; Karapondo, D.L.; Malicky, E.S.; Falkel, J.E.; Hagerman, F.C.; Hikida, R.S. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J. Appl. Physiol. 1991, 70, 631–640. [Google Scholar] [CrossRef]
- Taaffe, D.; Marcus, R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin. Physiol. 1997, 17, 311–324. [Google Scholar] [CrossRef]
- Neal, A.; Boldrin, L.; Morgan, J.E. The satellite cell in male and female, developing and adult mouse muscle: Distinct stem cells for growth and regeneration. PLoS ONE 2012, 7, e37950. [Google Scholar] [CrossRef] [Green Version]
- Seaborne, R.; Strauss, J.; Cocks, M.; Shepherd, S.; O’Brien, T.D.; Van Someren, K.A.; Bell, P.G.; Murgatroyd, C.; Morton, J.P.; Stewart, C.E.; et al. Methylome of human skeletal muscle after acute & chronic resistance exercise training, detraining & retraining. Sci. Data 2018, 5, 180213. [Google Scholar]
- Seaborne, R.A.; Strauss, J.; Cocks, M.; Shepherd, S.; O’Brien, T.D.; Van Someren, K.A.; Bell, P.G.; Murgatroyd, C.; Morton, J.P.; Stewart, C.E.; et al. Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Sci. Rep. 2018, 8, 1898. [Google Scholar] [CrossRef]
- Karunasena, N.; Han, T.S.; Mallappa, A.; Elman, M.; Merke, D.P.; Ross, R.J.; Daniel, E. Androgens correlate with increased erythropoiesis in women with congenital adrenal hyperplasia. Clin. Endocrinol. 2017, 86, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hicks, B.M.; Klil-Drori, A.; Yin, H.; Campeau, L.; Azoulay, L. Androgen Deprivation Therapy and the Risk of Anemia in Men with Prostate Cancer. Epidemiology 2017, 28, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Berlin, J.A.; Hannoush, P.; Haddad, G.; Dlewati, A.; Santanna, J.; Loh, L.; Lenrow, D.A.; Holmes, J.H.; et al. Effects of testosterone replacement in hypogonadal men. J. Clin. Endocrinol. Metab. 2000, 85, 2670–2677. [Google Scholar] [CrossRef] [PubMed]

| Sport | Male | Male | Male | Female | Finishing Position for Female Gold in Male Event |
|---|---|---|---|---|---|
| Athletics | Gold | Silver | Bronze | Gold | |
| 10,000 m run | 27.05.17 s | 27.05.64 s | 27.05.26 s | 29.17.45 s | 32nd |
| 100 m run | 9.81 s | 9.89 s | 9.91 s | 10.71 s | Would not have qualified for gold medal final |
| 400 m run | 43.03 s | 43.76 s | 43.85 s | 49.44 s | Would not have qualified for the gold medal final |
| 800 m run | 1:42:15 min | 1:42:61 min | 1:42:93 min | 1:55:28 min | Would not have qualified for the gold medal final |
| marathon | 2.08.44 h | 2.09.54 h | 2.10.05 h | 2.24.04 h | 90th |
| long jump | 8.38 m | 8.37 m | 8.29 m | 7.17 m | <12th (cut off at 7.82 m) |
| Triathlon | 1.45.01 h | 1.45.07 h | 1.45.43 h | 1.56.16 h | 50th |
| Swimming | |||||
| 100 m freestyle | 47.58 s | 47.8 s | 47.85 s | 52.70 s | 51st out of 57 in the heats |
| 400 m individual medley | 4.06.05 m | 4.06.75 m | 4.09.71 m | 4.26.36 m | 26th out of 27 in the heats |
| Canoe sprint | |||||
| K-1200 m (Kayak single) | 35.197 s | 35.362 s | 35.662 s | 39.864 s. | Would not have placed in the top 16 to make finals (cut off 38.061) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heather, A.K. Transwoman Elite Athletes: Their Extra Percentage Relative to Female Physiology. Int. J. Environ. Res. Public Health 2022, 19, 9103. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19159103
Heather AK. Transwoman Elite Athletes: Their Extra Percentage Relative to Female Physiology. International Journal of Environmental Research and Public Health. 2022; 19(15):9103. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19159103
Chicago/Turabian StyleHeather, Alison K. 2022. "Transwoman Elite Athletes: Their Extra Percentage Relative to Female Physiology" International Journal of Environmental Research and Public Health 19, no. 15: 9103. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19159103