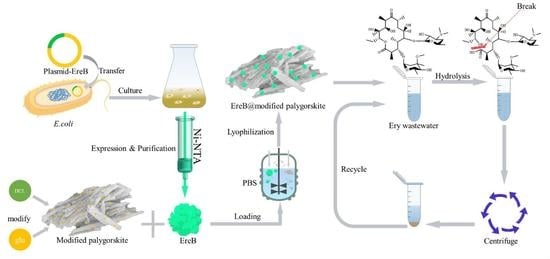
Immobilization of EreB on Acid-Modified Palygorskite for Highly Efficient Degradation of Erythromycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gene Cloning, Vector Construction, and Heterogeneous Expression
2.3. Palygorskite Modification by Acid and Glutaraldehyde
2.4. Synthesis of EreB@modified Palygorskite and Reaction Optimization
2.5. Characterization of EreB@modified Palygorskite
2.6. Determination of Enzyme Activity
2.7. Application of EreB@modified Palygorskite in the Erythromycin Reactor
3. Results and Discussion
3.1. Gene Cloning, Vector Construction, and Heterogeneous Expression of EreB
3.2. Optimization of Temperature, Enzyme, and Glutaraldehyde Loadings
3.3. Characterization of EreB@modified Palygorskite
3.4. Effect of pH and Temperature on Activity
3.5. Enzyme-Kinetic Parameters of EreB@modified Palygorskite
3.6. Reusability of EreB@modified Palygorskite and Verification of its Degradation Effect in Polluted Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Feng, J.; Liu, J.; Fu, W.; Li, X.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef]
- Rocha, C.S.; Kochi, L.Y.; Ribeiro, G.B.; Rocha, D.C.; Carneiro, D.N.M.; Gomes, M.P. Evaluating aquatic macrophytes for removing erythromycin from contaminated water: Floating or submerged? Int. J. Phytoremediat. 2021, 24, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Feng, M.; Chen, J.; Shen, W.; Zhang, S. Occurrence of antibiotics and antibiotic resistance genes and their correlations in river-type drinking water source, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 42339–42352. [Google Scholar] [CrossRef]
- Chu, L.; Chen, D.; Wang, J.; Yang, Z.; Shen, Y. Degradation of antibiotics and antibiotic resistance genes in erythromycin fermentation residues using radiation coupled with peroxymonosulfate oxidation. Waste Manag. 2019, 96, 190–197. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Shen, D.; Gu, X.; Zheng, Y.; Delgado-Moreno, L.; Jia, W.; Ye, Q.; Wang, W. The fate of erythromycin in soils and its effect on soil microbial community structure. Sci. Total Environ. 2022, 820, 153373. [Google Scholar] [CrossRef]
- Cai, C.; Hua, Y.; Li, H.; Li, L.; Dai, L.; Liu, H.; Dai, X. Hydrothermal treatment of erythromycin fermentation residue: Harmless performance and bioresource properties. Resour. Conserv. Recy. 2020, 161, 104952. [Google Scholar] [CrossRef]
- Tang, R.; Yuan, S.; Chen, F.; Zhan, X.; Wang, W.; Hu, Z. Effects of roxarsone and sulfadiazine on biogas production and their degradation during anaerobic digestion. Int. Biodeter. Biodegr. 2019, 140, 113–118. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Z.; Deng, L.; Niu, D.; Fan, B.; Huhe, T.; Li, Z.; Zhang, J.; Li, C. Biodegradation of erythromycin by Delftia lacustris RJJ-61 and characterization of its erythromycin esterase. J. Basic Microbiol. 2021, 61, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Geng, J.; Ma, L.; Sun, X.; Qi, H.; Wu, Y.; Zhang, R. Changes in antibiotic resistance genotypes and phenotypes after two typical sewage disposal processes. Chemosphere 2022, 291 Pt 2, 132833. [Google Scholar] [CrossRef]
- Zielinski, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and functional insights into esterase-mediated macrolide resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Morar, M.; Pengelly, K.; Koteva, K.; Wright, G.D. Mechanism and diversity of the erythromycin esterase family of enzymes. Biochemistry 2012, 51, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Biskri, L.; Mazel, D. Erythromycin esterase gene ere(A) is located in a functional gene cassette in an unusual class 2 integron. Antimicrob. Agents Chemother. 2003, 47, 3326–3331. [Google Scholar] [CrossRef]
- Nakamura, A.; Nakazawa, K.; Miyakozawa, T.; Mizukoshi, S.; Tsurubuchi, K.; Nakagawa, M.; O’Hara, K.; Sawai, T. Macrolide esterase-producing Escherichia coli clinically isolated in Japan. J. Antibiot. 2000, 53, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Cha, C.J.; Cerniglia, C.E. Purification and characterization of an erythromycin esterase from an erythromycin-resistant Pseudomonas sp. Fems. Microbiol. Lett. 2002, 210, 239–244. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Silva, V.C.; Batista, T.S.; Ramos, I.B.M.; Barros, T.R.B.; de Sousa, B.V. Organophilization Process and Characterization of Palygorskite Clay (Attapulgite). Mater. Sci. Forum. 2016, 881, 218–223. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Wang, X. Influence of differently modified palygorskites in the immobilization of a lipase. J. Mol. Catal. B-Enzym. 2008, 55, 49–54. [Google Scholar] [CrossRef]
- Liu, F.G.; Zhao, L.Z.; An, N.; Tong, D.S.; Yu, W.H.; Zhou, C.H. Modification of inorganic porous materials as gene vectors: An overview. J. Porous Mat. 2015, 22, 927–937. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Juang, R.-S. Use of chitosan–clay composite as immobilization support for improved activity and stability of β-glucosidase. Biochem. Eng. J. 2007, 35, 93–98. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Sheng, G.; Hu, L. The study on effective immobilization of lipase on functionalized bentonites and their properties. J. Mol. Catal. B-Enzym. 2013, 95, 9–15. [Google Scholar] [CrossRef]
- Tan, Z.; Zhou, J.; Li, X.; Ren, S.; You, Q.; Bilal, M. Immobilization of a cold-adaptive recombinant Penicillium cyclopium lipase on modified palygorskite for biodiesel preparation. Biomass Convers Bior. 2021. [Google Scholar] [CrossRef]
- Qinhua, F.; Xiaofan, Z. Study on Immobilization of Plant-esterase on Attapulgite. Environ. Sci. Technol. 2015, 38, 23–28, 54. [Google Scholar]
- Xavier, K.C.M.; Santos, M.R.; Oliveira, M.E.R.; Carvalho, M.W.N.C.; Osajima, J.; Filho, E.C.D.S. Effects of acid treatment on the clay palygorskite: XRD, surface area, morphological and chemical composition. Mater. Res.-Ibero.-Am. J. 2014, 17 (Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Hiol, A.; Jonzo, M.D.; Druet, D.; Comeau, L. Production, purification and characterization of an extracellular lipase from Mucor hiemalis f. hiemalis. Enzyme Microb. Tech. 1999, 25, 80–87. [Google Scholar] [CrossRef]
- López-Gallego, F.; Guisan, J.M.; Betancor, L. Immobilization of Enzymes on Supports Activated with Glutaraldehyde: A Very Simple Immobilization Protocol. In Immobilization of Enzymes and Cells: Methods and Protocols; Guisan, J.M., Bolivar, J.M., López-Gallego, F., Rocha-Martín, J., Eds.; Springer: New York, NY, USA, 2020; pp. 119–127. [Google Scholar]
- Lucarini, A.C.; Kilikian, B.V. Comparative study of Lowry and Bradford methods: Interfering substances. Biotechnol. Tech. 1999, 13, 149–154. [Google Scholar] [CrossRef]
- de Cazes, M.; Belleville, M.P.; Petit, E.; Salomo, M.; Bayer, S.; Czaja, R.; De Gunzburg, J.; Sanchez-Marcano, J. Erythromycin degradation by esterase (EreB) in enzymatic membrane reactors. Biochem. Eng. J. 2016, 114, 70–78. [Google Scholar] [CrossRef]
- Benitez-Mateos, A.I.; San Sebastian, E.; Rios-Lombardia, N.; Moris, F.; Gonzalez-Sabin, J.; Lopez-Gallego, F. Asymmetric Reduction of Prochiral Ketones by Using Self-Sufficient Heterogeneous Biocatalysts Based on NADPH-Dependent Ketoreductases. Chemistry 2017, 23, 16843–16852. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Z.; Deng, L.; Niu, D.; Huhetaoli; Li, Z.; Dong, L.; Zhang, J.; Zhang, R.; Li, C. Degradation of Erythromycin by a Novel Fungus, Penicillium oxalicum RJJ-2, and the Degradation Pathway. Waste Biomass Valori. 2021, 12, 4513–4523. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisan, J.M.; Fernandez-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, T.; Borchert, T.W.; Nielsen, V.S.; Skagerlind, P.; Gibson, K.; Wenger, K.; Hatzack, F.; Nilsson, L.D.; Salmon, S.; Pedersen, S.; et al. Industrial Enzymes. In White Biotechnology; Ulber, R., Sell, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 59–131. [Google Scholar]
- Wu, X.; Yang, C.; Ge, J.; Liu, Z. Polydopamine tethered enzyme/metal-organic framework composites with high stability and reusability. Nanoscale 2015, 7, 18883–18886. [Google Scholar] [CrossRef]
- Bedade, D.K.; Muley, A.B.; Singhal, R.S. Magnetic cross-linked enzyme aggregates of acrylamidase from Cupriavidus oxalaticus ICTDB921 for biodegradation of acrylamide from industrial waste water. Bioresour. Technol. 2019, 272, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Wang, T.; Xiao, Y.; Huo, Q.; Liu, Y. Immobilization of Bacillus subtilis lipase on a Cu-BTC based hierarchically porous metal-organic framework material: A biocatalyst for esterification. Dalton. Trans. 2016, 45, 6998–7003. [Google Scholar] [CrossRef]
- Sarbu, A.; de Pinho, M.N.; Freixo, M.D.R.; Goncalves, F.; Udrea, I. New method for the covalent immobilization of a xylanase by radical grafting of acrylamide on cellulose acetate membranes. Enzym. Microb. Tech. 2006, 39, 125–130. [Google Scholar] [CrossRef]
- Bertrand, E.; Pierre, G.; Delattre, C.; Gardarin, C.; Bridiau, N.; Maugard, T.; Strancar, A.; Michaud, P. Dextranase immobilization on epoxy CIM((R)) disk for the production of isomaltooligosaccharides from dextran. Carbohyd. Polym. 2014, 111, 707–713. [Google Scholar] [CrossRef]
- Xia, H.; Li, Z.; Zhong, X.; Li, B.; Jiang, Y.; Jiang, Y. HKUST-1 catalyzed efficient in situ regeneration of NAD+ for dehydrogenase mediated oxidation. Chem. Eng. Sci. 2019, 203, 43–53. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Ladole, M.R.; Pokale, P.B.; Patil, S.S.; Belokar, P.G.; Pandit, A.B. Laccase immobilized peroxidase mimicking magnetic metal organic frameworks for industrial dye degradation. Bioresour. Technol. 2020, 317, 124035. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Y.; Liu, X.; Zhang, H. Solvent-Free Synthetic Fe3O4@ZIF-8 Coated Lipase as a Magnetic-Responsive Pickering Emulsifier for Interfacial Biocatalysis. Catal. Lett. 2020, 150, 3608–3616. [Google Scholar] [CrossRef]
- Ahmed, I.N.; Yang, X.L.; Dubale, A.A. Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks. Bioresour. Technol. 2018, 270, 377–382. [Google Scholar] [CrossRef]







| Catalyst | KM (mM) | Vmax (mM min−1) | kcat (min−1) | kcat/KM (min−1 mM−1) |
|---|---|---|---|---|
| Free EreB | 438.49 | 0.11 | 1202.75 | 2.74 |
| Immobilized EreB | 1129.04 | 0.12 | 1371.21 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, S.; Li, C.; Yu, Y.; Niu, D.; Zhu, J.; Yin, D.; Wang, C.; Zhang, W.; Jiang, X.; Ren, J. Immobilization of EreB on Acid-Modified Palygorskite for Highly Efficient Degradation of Erythromycin. Int. J. Environ. Res. Public Health 2022, 19, 11064. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191711064
Ni S, Li C, Yu Y, Niu D, Zhu J, Yin D, Wang C, Zhang W, Jiang X, Ren J. Immobilization of EreB on Acid-Modified Palygorskite for Highly Efficient Degradation of Erythromycin. International Journal of Environmental Research and Public Health. 2022; 19(17):11064. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191711064
Chicago/Turabian StyleNi, Shensheng, Chunyu Li, Yicheng Yu, Dongze Niu, Jie Zhu, Dongmin Yin, Chongqing Wang, Wenfan Zhang, Xingmei Jiang, and Jianjun Ren. 2022. "Immobilization of EreB on Acid-Modified Palygorskite for Highly Efficient Degradation of Erythromycin" International Journal of Environmental Research and Public Health 19, no. 17: 11064. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191711064