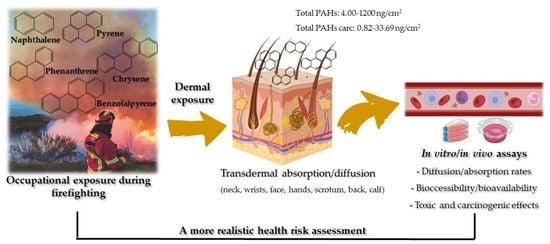
Exposure to PAHs during Firefighting Activities: A Review on Skin Levels, In Vitro/In Vivo Bioavailability, and Health Risks
Abstract
:1. Introduction
2. Methods
3. Concentrations of PAHs on the Skin of Firefighters
3.1. Levels of Total PAHs
3.2. Levels of Pyrene
3.3. Levels of Carcinogenic PAHs
4. Dermal In Vitro/In Vivo Studies
4.1. Dermal Absorption
4.2. Dermal Bioaccessibility and Bioavailability
4.3. Toxicological and Carcinogenic Dermal Risks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO: Geneva, Switzerland, 2021; Volume xxi. [Google Scholar]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.; Zhang, H.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Hayakawa, K.; Toriba, A.; Wei, Y.; et al. Assessing Approaches of Human Inhalation Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3124. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.U.; Chishtie, F.; Shahid, I.; Mahmud, T.; Hussain, R. Traffic-and Industry-Related Air Pollution Exposure Assessment in an Asian Megacity. Clean–Soil Air Water 2018, 46, 1600773–1600793. [Google Scholar] [CrossRef]
- Munyeza, C.F.; Rohwer, E.R.; Forbes, P.B.C. A review of monitoring of airborne polycyclic aromatic hydrocarbons: An African perspective. Trends Environ. Anal. Chem. 2019, 24, e00070–e00081. [Google Scholar] [CrossRef]
- Yan, D.; Wu, S.; Zhou, S.; Tong, G.; Li, F.; Wang, Y.; Li, B. Characteristics, sources and health risk assessment of airborne particulate PAHs in Chinese cities: A review. Environ. Pollut. 2019, 248, 804–814. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Abolhasani, R.; Araghi, F.; Tabary, M.; Aryannejad, A.; Mashinchi, B.; Robati, R.M. The impact of air pollution on skin and related disorders: A comprehensive review. Dermatol. Ther. 2021, 34, e14840–e14851. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759–776. [Google Scholar]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors–Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530–101544. [Google Scholar] [CrossRef]
- Peng, F.; Tsuji, G.; Zhang, J.-Z.; Chen, Z.; Furue, M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019, 132, 105063–105067. [Google Scholar] [CrossRef]
- Damevska, K.; Nikolovska, S.; Kazandjieva, J.; Kotevska, B.; Bocheva, G. Skin and pollution. In Advances in Integrative Dermatology; Frana, K., Lotti, T., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2019; pp. 379–392. [Google Scholar]
- Alalaiwe, A.; Lin, Y.-K.; Lin, C.-H.; Wang, P.-W.; Lin, J.-Y.; Fang, J.-Y. The absorption of polycyclic aromatic hydrocarbons into the skin to elicit cutaneous inflammation: The establishment of structure–permeation and in silico–in vitro–in vivo relationships. Chemosphere 2020, 255, 126955–126969. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Zeng, D.; Kang, Y.; Lin, X.; Sun, N.; Li, C.; Zhu, M.; Chen, Z.; Man, Y.B.; Li, H. Dermal bioaccessibility and absorption of polycyclic aromatic hydrocarbons (PAHs) in indoor dust and its implication in risk assessment. Environ. Pollut. 2020, 264, 114829–114838. [Google Scholar] [CrossRef] [PubMed]
- Mertsching, H.; Weimer, M.; Kersen, S.; Brunner, H. Human skin equivalent as an alternative to animal testing. GMS Krankenhhyg. Interdiszip. 2008, 3, Doc11–Doc15. [Google Scholar] [PubMed]
- Kamal, A.; Cincinelli, A.; Fau-Martellini, T.; Martellini, T.; Fau-Malik, R.N.; Malik, R.N. A review of PAH exposure from the combustion of biomass fuel and their less surveyed effect on the blood parameters. Environ. Sci. Pollut. Res. Int. 2015, 22, 4076–4098. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists. Polycyclic Aromatic Hydrocarbons (PAHs) Biologic Exposure Indices (BEI) Cincinnati; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2005. [Google Scholar]
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC Monogr Eval Carcinog Risks Hum: Lyon, France, 2010; Volume 92.
- International Agency for Research on Cancer. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Monogr Eval Carcinog Risks Hum: Lyon, France, 2002; Volume 82.
- World Health Organization. Review of Evidence on Health Aspects of Air Pollution–REVIHAAP Project: Final Technical Report; World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35–63. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann. Rev. Pharmacol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- World Health Organization. Polycyclic Aromatic Hydrocarbons (PAHs); World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2000; Volume 5.9. [Google Scholar]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef]
- Demers, P.A.; DeMarini, D.M.; Fent, K.W.; Glass, D.C.; Hansen, J.; Adetona, O.; Andersen, M.H.G.; Freeman, L.E.B.; Caban-Martinez, A.J.; Daniels, R.D.; et al. Carcinogenicity of occupational exposure as a firefighter. Lancet Oncol. 2022, 23, 985–986. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Painting, Firefighting, and Shiftwork; IARC Monogr Eval Carcinog Risks Hum: Lyon, France, 2010; Volume 98.
- Barros, B.; Oliveira, M.; Morais, S. Firefighters’ occupational exposure: Contribution from biomarkers of effect to assess health risks. Environ. Int. 2021, 156, 106704–106725. [Google Scholar] [CrossRef]
- Sjöström, M.; Julander, A.; Strandberg, B.; Lewné, M.; Bigert, C. Airborne and dermal exposure to polycyclic aromatic hydrocarbons, volatile organic compounds, and particles among firefighters and police investigators. Ann. Work Expo. Health 2019, 63, 533–545. [Google Scholar] [CrossRef]
- Hwang, J.; Xu, C.; Agnew, R.J.; Clifton, S.; Malone, T.R. Health risks of structural firefighters from exposure to polycyclic aromatic hydrocarbons: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 4209. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Magalhães, C.P.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; do Carmo Pereira, M.; Morais, S. Individual and cumulative impacts of fire emissions and tobacco consumption on wildland firefighters’ total exposure to polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2017, 334, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Beitel, S.C.; Flahr, L.M.; Hoppe-Jones, C.; Burgess, J.L.; Littau, S.R.; Gulotta, J.; Moore, P.; Wallentine, D.; Snyder, S.A. Assessment of the toxicity of firefighter exposures using the PAH CALUX bioassay. Environ. Int. 2020, 135, 105207–105215. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Costa, S.; Vaz, J.; Fernandes, A.; Slezakova, K.; Delerue-Matos, C.; Teixeira, J.P.; Carmo Pereira, M.; Morais, S. Firefighters exposure to fire emissions: Impact on levels of biomarkers of exposure to polycyclic aromatic hydrocarbons and genotoxic/oxidative-effects. J. Hazard. Mater. 2020, 383, 121179–121189. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of firefighters to particulates and polycyclic aromatic hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef]
- Fent, K.W.; Eisenberg, J.; Snawder, J.; Sammons, D.; Pleil, J.D.; Stiegel, M.A.; Mueller, C.; Horn, G.P.; Dalton, J. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann. Occup. Hyg. 2014, 58, 830–845. [Google Scholar]
- Keir, J.L.A.; Akhtar, U.S.; Matschke, D.M.J.; Kirkham, T.L.; Chan, H.M.; Ayotte, P.; White, P.A.; Blais, J.M. Elevated Exposures to Polycyclic Aromatic Hydrocarbons and Other Organic Mutagens in Ottawa Firefighters Participating in Emergency, On-Shift Fire Suppression. Environ. Sci. Technol. 2017, 51, 12745–12755. [Google Scholar] [CrossRef]
- Wingfors, H.; Nyholm, J.R.; Magnusson, R.; Wijkmark, C.H. Impact of fire suit ensembles on firefighter pah exposures as assessed by skin deposition and urinary biomarkers. Ann. Work Expo. Health 2018, 62, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Fent, K.W.; Toennis, C.; Sammons, D.; Robertson, S.; Bertke, S.; Calafat, A.M.; Pleil, J.D.; Wallace, M.A.G.; Kerber, S.; Smith, D.; et al. Firefighters’ absorption of PAHs and VOCs during controlled residential fires by job assignment and fire attack tactic. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 338–349. [Google Scholar] [CrossRef]
- Rossbach, B.; Wollschläger, D.; Letzel, S.; Gottschalk, W.; Muttray, A. Internal exposure of firefighting instructors to polycyclic aromatic hydrocarbons (PAH) during live fire training. Toxicol. Lett. 2020, 331, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Pedersen, J.E.; Pedersen, P.B.; Clausen, P.A.; Løhr, M.; Kermanizadeh, A.; Loft, S.; Ebbehøj, N.E.; Hansen, Å.M.; et al. Assessment of polycyclic aromatic hydrocarbon exposure, lung function, systemic inflammation, and genotoxicity in peripheral blood mononuclear cells from firefighters before and after a work shift. Environ. Mol. Mutagen. 2018, 59, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, J.; Mäkelä, M.; Mikkola, J.; Huttu, I. Fire fighting trainers’ exposure to carcinogenic agents in smoke diving simulators. Toxicol. Lett. 2010, 192, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Banks, A.P.W.; Thai, P.K.; Engelsman, M.; Wang, X.; Osorio, A.F.; Mueller, J.F. Characterising the exposure of Australian firefighters to polycyclic aromatic hydrocarbons generated in simulated compartment fires. Int. J. Hyg. Environ. Health 2020, 231, 113637–113644. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Clausen, P.A.; Pedersen, J.E.; Løhr, M.; Kermanizadeh, A.; Loft, S.; Ebbehøj, N.; Hansen, Å.M.; Pedersen, P.B.; et al. Association between polycyclic aromatic hydrocarbon exposure and peripheral blood mononuclear cell DNA damage in human volunteers during fire extinction exercises. Mutagenesis 2017, 33, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fent, K.W.; Alexander, B.M.; Roberts, J.; Robertson, S.; Toennis, C.A.; Sammons, D.L.; Bertke, S.J.; Kerber, S.; Smith, D.L.; Horn, G.P. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg. 2017, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Shaw, L.; Shaw, D.S.; Gallea, M.; vanden Enden, L.; House, R.; Verma, D.K.; Britz McKibbin, P.; McCarry, B.E. Evaluation of Firefighter Exposure to Wood Smoke during Training Exercises at Burn Houses. Environ. Sci. Technol. 2016, 50, 1536–1543. [Google Scholar] [CrossRef]
- Strandberg, B.; Julander, A.; Sjöström, M.; Lewné, M.; Hatice, K.A.; Bigert, C. An improved method for determining dermal exposure to polycyclic aromatic hydrocarbons. Chemosphere 2018, 198, 274–280. [Google Scholar] [CrossRef]
- Mayer, A.C.; Fent, K.W.; Wilkinson, A.; Chen, I.C.; Kerber, S.; Smith, D.L.; Kesler, R.M.; Horn, G.P. Characterizing exposure to benzene, toluene, and naphthalene in firefighters wearing different types of new or laundered PPE. Int. J. Hyg. Environ. Health 2022, 240, 113900–113909. [Google Scholar] [CrossRef]
- Mayer, A.C.; Horn, G.P.; Fent, K.W.; Bertke, S.J.; Kerber, S.; Kesler, R.M.; Newman, H.; Smith, D.L. Impact of select PPE design elements and repeated laundering in firefighter protection from smoke exposure. J. Occup. Environ. Hyg. 2020, 17, 505–514. [Google Scholar] [CrossRef]
- Fent, K.W.; LaGuardia, M.; Luellen, D.; McCormick, S.; Mayer, A.; Chen, I.C.; Kerber, S.; Smith, D.; Horn, G.P. Flame retardants, dioxins, and furans in air and on firefighters’ protective ensembles during controlled residential firefighting. Environ. Int. 2020, 140, 105756–105765. [Google Scholar] [CrossRef] [PubMed]
- Banks, A.P.W.; Wang, X.; Engelsman, M.; He, C.; Osorio, A.F.; Mueller, J.F. Assessing decontamination and laundering processes for the removal of polycyclic aromatic hydrocarbons and flame retardants from firefighting uniforms. Environ. Res. 2021, 194, 110616–110624. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, S.; Szewczyńska, M. PAH contamination of firefighter protective clothing and cleaning effectiveness. Fire Saf. J. 2022, 131, 103610–103621. [Google Scholar] [CrossRef]
- Bourgart, E.; Barbeau, D.; Marques, M.; von Koschembahr, A.; Béal, D.; Persoons, R.; Leccia, M.-T.; Douki, T.; Maitre, A. A realistic human skin model to study benzo[a]pyrene cutaneous absorption in order to determine the most relevant biomarker for carcinogenic exposure. Arch. Toxicol. 2019, 93, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.A.; Kriech, A.J.; Mackerer, C.R. Percutaneous Absorption of Polycyclic Aromatic Compounds from Bitumen Fume Condensate. J. Occup. Environ. Hyg. 2007, 4, 137–143. [Google Scholar] [CrossRef]
- Moody, R.P.; Nadeau, B.; Chu, I. In vivo and in vitro dermal absorption of benzo[a]pyrene in rat, guinea pig, human and tissue-cultured skin. J. Dermatol. Sci. 1995, 9, 48–58. [Google Scholar] [CrossRef]
- Ng, K.M.; Chu, I.; Bronaugh, R.L.; Franklin, C.A.; Somers, D.A. Percutaneous absorption/metabolism of phenanthrene in the hairless guinea pig: Comparison of in vitro and in vivo results. Fundam. Appl. Toxicol. 1991, 16, 517–524. [Google Scholar] [CrossRef]
- Sartorelli, P.; Aprea, C.; Cenni, A.; Novelli, M.T.; Orsi, D.; Palmi, S.; Matteucci, G. Prediction of percutaneous absorption from physicochemical data: A model based on data of in vitro experiments. Ann. Occup. Hyg. 1998, 42, 267–276. [Google Scholar] [CrossRef]
- Moody, R.P.; Tytchino, A.V.; Yip, A.; Petrovic, S. A Novel “By Difference” Method for Assessing Dermal Absorption of Polycyclic Aromatic Hydrocarbons from Soil at Federal Contaminated Sites. J. Toxicol. Environ. Health A 2011, 74, 1294–1303. [Google Scholar] [CrossRef]
- Roy, T.A.; Krueger, A.J.; Taylor, B.B.; Mauro, D.M.; Goldstein, L.S. Studies Estimating the Dermal Bioavailability of Polynuclear Aromatic Hydrocarbons from Manufactured Gas Plant Tar-Contaminated Soils. Environ. Sci. Technol. 1998, 32, 3113–3117. [Google Scholar] [CrossRef]
- Roy, T.A.; Krueger, A.J.; Mackerer, C.R.; Neil, W.; Arroyo, A.M.; Yang, J.J. SAR models for estimating the percutaneous absorption of polynuclear aromatic hydrocarbons. SAR QSAR Environ. Res. 1998, 9, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Kadry, A.M.; Skowronski, G.A.; Turkall, R.M.; Abdel-Rahman, M.S. Comparison between oral and dermal bioavailability of soil-adsorbed phenanthrene in female rats. Toxicol. Lett. 1995, 78, 153–163. [Google Scholar] [CrossRef]
- Daniel, P.M.; Pratt, O.E.; Prichard, M.M.L. Metabolism of Labelled Carcinogenic Hydrocarbons in Rats. Nature 1967, 215, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.M.; Yoshida, A.; Epstein, S.S. Uptake and excretion of benzo[a]pyrene and its metabolites by the rat pancreas. Drug Metab. Dispos. 1979, 7, 44–48. [Google Scholar] [PubMed]
- Stroo, H.F.; Roy, T.A.; Liban, C.B.; Kreitinger, J.P. Dermal bioavailability of benzo[a]pyrene on lampblack: Implications for risk assessment. Environ. Toxicol. Chem. 2005, 24, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, N.D.; Haney, J.T.; Hoeger, G.C.; Meyer, A.K.; Magee, B.H. Oral and Dermal Bioavailability Studies of Polycyclic Aromatic Hydrocarbons from Soils Containing Weathered Fragments of Clay Shooting Targets. Environ. Sci. Technol. 2021, 55, 6897–6906. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency Office of Health and Environmental Assessment. Dermal Exposure Assessment: Principles and Applications; EPA/600/8−91/011B; Office of Health and Environmental Assessment: Washington, DC, USA, 1992.
- Potratz, S.; Jungnickel, H.; Grabiger, S.; Tarnow, P.; Otto, W.; Fritsche, E.; von Bergen, M.; Luch, A. Differential cellular metabolite alterations in HaCaT cells caused by exposure to the aryl hydrocarbon receptor-binding polycyclic aromatic hydrocarbons chrysene, benzo[a]pyrene and dibenzo[a,l]pyrene. Toxicol. Rep. 2016, 3, 763–773. [Google Scholar] [CrossRef]
- Hall, M.; Grover, P.L. Polycyclic Aromatic Hydrocarbons: Metabolism, Activation and Tumour Initiation. In Chemical Carcinogenesis and Mutagenesis I; Cooper, C.S., Grover, P.L., Eds.; Springer: Berlin, Germany, 1990; pp. 327–372. [Google Scholar]
- Cavalieri, E.L.; Rogan, E.G. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica 1995, 25, 677–688. [Google Scholar] [CrossRef]
- Penning, T.M.; Ohnishi, S.T.; Ohnishi, T.; Harvey, R.G. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem. Res. Toxicol. 1996, 9, 84–92. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Sivak, A.; Niemeier, R.; Lynch, D.; Beltis, K.; Simon, S.; Salomon, R.; Latta, R.; Belinky, B.; Menzies, K.; Lunsford, A.; et al. Skin carcinogenicity of condensed asphalt roofing fumes and their fractions following dermal application to mice. Cancer Lett. 1997, 117, 113–123. [Google Scholar] [CrossRef]
- Hall, M.; Grover, P.L. Stereoselective aspects of the metabolic activation of benzo[a]pyrene by human skin in vitro. Chem. Biol. Interact. 1988, 64, 281–296. [Google Scholar] [CrossRef]
- Siddens, L.K.; Larkin, A.; Krueger, S.K.; Bradfield, C.A.; Waters, K.M.; Tilton, S.C.; Pereira, C.B.; Löhr, C.V.; Arlt, V.M.; Phillips, D.H.; et al. Polycyclic aromatic hydrocarbons as skin carcinogens: Comparison of benzo[a]pyrene, dibenzo[def,p]chrysene and three environmental mixtures in the FVB/N mouse. Toxicol. Appl. Pharm. 2012, 264, 377–386. [Google Scholar] [CrossRef]
- Stolpmann, K.; Brinkmann, J.; Salzmann, S.; Genkinger, D.; Fritsche, E.; Hutzler, C.; Wajant, H.; Luch, A.; Henkler, F. Activation of the aryl hydrocarbon receptor sensitises human keratinocytes for CD95L- and TRAIL-induced apoptosis. Cell Death Dis. 2012, 3, e388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeur, J.; Belaïdi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Pieterse, B.; Felzel, E.; Winter, R.; van der Burg, B.; Brouwer, A. PAH-CALUX, an Optimized Bioassay for AhR-Mediated Hazard Identification of Polycyclic Aromatic Hydrocarbons (PAHs) as Individual Compounds and in Complex Mixtures. Environ. Sci. Technol. 2013, 47, 11651–11659. [Google Scholar] [CrossRef]




| Model (Dosing Vehicle) | PAHs | Units | Results | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Hairless guinea pig (acetone) | Phe | Mean ± SEM (%) | (a) Permeation in vitro flow-through cells (balanced salt solution, 6.6 µg/cm2) Receptor fluid: (6 h) 2.4 ± 0.5; (12 h) 6.4 ± 1.2; (18 h) 8.3 ± 1.3; (24 h) 8.7 ± 2.0 Dose remaining in skin: 59.7 ± 2.0; Total dose: 68.5 ± 2.0; Skin wash: 10.3 ± 2.0; Recovered dose: 78.8 ± 2.1 (b) Permeation in vitro flow-through cells (balanced salt solution, bovine serum albumin, 6.6 µg/cm2) Receptor fluid:(6 h) 38.9 ± 8.1; (12 h) 63.6 ± 8.8; (18 h) 73.4 ± 8.5; (24 h) 78.0 ± 8.6 Dose remaining in skin: 11.8 ± 0.1; Total dose: 89.7 ± 8.7; Skin wash: 3.5 ± 1.2; Recovered dose: 93.3 ± 8.4 (c) Permeation in vitro flow-through cells (water, bovine serum albumin, 15.2 µg/cm2) Receptor fluid: (6 h) 20.8 ± 0.5; (12 h) 41.4 ± 0.9; (18 h) 52.1 ± 2.5; (24 h) 57.4 ± 2.7 Dose remaining in skin: 8.8 ± 1.0; Total dose: 66.2 ± 3.3; Skin wash: 2.2 ± 0.2; Recovered dose: 68.4 ± 3.5 (d) Permeation in vitro flow-through cells (balanced salt solution, bovine serum albumin, 15.2 µg/cm2) Receptor fluid: (6 h) 33.7 ± 7.3; (12 h) 58.1 ± 7.7; (18 h) 67.2 ± 6.5; (24 h) 71.3 ± 5.7 Dose remaining in skin: 7.8 ± 2.8; Total dose: 79.1 ± 3.6; Skin wash: 1.6 ± 0.4; Recovered dose: 80.7 ± 3.2 | In vitro percutaneous absorption of Phe; Penetration through pig skin controlled more by the passive rate of diffusion than by metabolism. | [55] |
| Rat, guinea pig, and human abdomen skin (acetone) | BaP | Mean ± SD (%) | (a) In vitro recoveries after topical applications with BaP Activity (radioactive soap, water washes, 24 h): (Rat) 5.3 ± 0.32; (Guinea pig) 45.9 ± 3.95; (Human, 32 y) 74.0 ± 6.94; (Human, 50 y) 42.9 ± 5.05 Activity (methanol extracts, 48 h): (Rat) 21.1 ± 5.53; (Guinea pig) 4.9 ± 1.47; (Human, 32 y) 13.5 ± 2.11; (Human, 50 y) 35.2 ± 8.11 Activity (skin digest): (Rat) 22.9 ± 5.24; (Guinea pig) 18.2 ± 4.16; (Human, 32 y) 6.6 ± 2.55; (Human, 50 y) 7.3 ± 0.81 Activity (methanol extract, skin digest): (Rat) 44.0 ± 10.35; (Guinea pig) 23.1 ± 2.76; (Human, 32 y) 20.1 ± 4.62; (Human, 50 y) 42.5 ± 8.71 | BaP is well absorbed through animal and human skin; Assessment of total exposure to BaP should consider the dermal route. | [54] |
| (µg/cm2) | (b) In vitro cumulative absorption of BaP topical application (48 h) (Rat) 5.6 ± 0.10; (Guinea pig) 3.7 ± 0.18; (Human, 32 y) 0.3 ± 0.07; (Human, 50 y) 0.1 ± 0.05 | ||||
| (µg/cm2/h) | (c) In vitro maximum rate of skin permeation (Rat) 0.38 ± 0.028; (Guinea pig) 0.42 ± 0.031; (Human, 32 y) 0.02 ± 0.009; (Human, 50 y) 0.01 ± 0.003 | ||||
| (%) | (d) In vivo total (urinary, fecal, and tissue) dermal absorption of BaP (Rat) 69.4 ± 7.59; (Guinea pig) 67.8 ± 9.33 | ||||
| Monkey skin (acetone) | 8 PAHs | Mean ± SD (cm/h) | Permeability constants Naph: (5.12 ± 2.88) × 10−3; Ace: (6.33 ± 4.81) × 10−3; Flu: (6.26 ± 4.74) × 10−3; Phe: (1.96 ± 1.14) × 10−3; Ant: (3.44 ± 3.09) × 10−3; Pyr: (1.69 ± 1.36) × 10−3; Chry: (0.22 ± 0.14) × 10−3; BaA: (0.15 ± 0.08) × 10−3 | KOW values correlated with the permeability constant (r = 0.90, p < 0.001) and the lag time (r = 0.81, p < 0.01); A multiple linear regression model between permeability constants, KOW, and water solubility was reported (p < 0.0001). | [56] |
| Human back skin (6% aqueous solution of polyoxyethylene 20 oleyl ether) | 18 PAHs | Mean ± SD (ng/cm2/h) | Directly measured values for flux: Ant 6.5 ± 0.9; Fln/Pyr 1.8 ± 0.3; 3–6 ring PAC: 120 ± 30 | High molecular weight compounds presented a reduced dermal penetration flux value. | [53] |
| % | Applied dose absorbed: Ant 5.3; Fln/Pyr 3.3; 3–6 ring PAHs: 1.8 | ||||
| (ng/cm2/h) | Dermal penetration flux values: Naph: 24; Acen: 0.094; Ace: 11; Flu: 37; Phe: 20; Ant: 6.5; Fln: 1.5; Pyr: 1.1; B(a)A: 0.23; Triphenylene: 0.16; Chry: 0.21; B(b)F: 0.035; B(k)F: 0.0044; BeP: 0.062; BaP: 0.016; Ind: 0.0013; DB(a,h)A: 0.0023; B(ghi)P: 0.0075 | ||||
| Human breast skin (acetone) | 11 PAHs | Mean ± SD (%) | In vitro dermal absorption rates in Bronaugh flow-through diffusion cells (24 h skin soap washes) (a) without soil: Phe 88.3 ± 4.83; Fln 82.6 ± 8.09; Pyr 82.5 ± 7.32; BaA 82.3 ± 8.70; Chry 81.5 ± 11.44; B(b)F 77.6 ± 7.49; B(k)F 81.0 ± 9.67; BaP 70.8 ± 5.73; DB(a,h)A 76.7 ± 15.36; B(ghi)P 75.2 ± 9.53; Ind 78.3 ± 7.17 (b) with soil: Phe 60.8 ± 7.75; Fln 49.9 ± 3.70; Pyr 48.7 ± 5.63; BaA 26.4 ± 8.09; Chry 33.5 ± 12.40 | High molecular weight compounds presented reduced dermal absorption. | [57] |
| Synthetic human skin (simulated artificial sweat and sebum mixture) | 4 PAHs | Value or Range (µg/L) | Skin absorption rates (up to 9 h post-exposure): Naph 0.22–1.84; Phe 0.24–2.30; Pyr 0.32–0.92; BaP 0.05–0.08 Intracellular levels: Naph 0.26, 0.76; Phe 0.47, 0.92; Pyr 0.45; BaP 0.08, 0.13 Residual levels: Naph 0.61, 1.13; Phe 0.63, 1.45; Pyr 1.13, 1.14; BaP 0.12 | Low molecular weight PAHs were more easily absorbed by skin cells than heavier compounds; | [14] |
| % | Total dermal penetration: Naph 76.4, 79.9; Phe 72.6, 73.3; Pyr 52.2, 38.7; BaP 8.30, 9.07 Total dermal absorption: Naph 84.6, 87.5; Phe 82.0, 82.5; Pyr 61.2, 43.2; BaP 10.9, 9.87 Loss ratio: Naph 6.19, 1.25; Phe 5.44, 3.00; Pyr 16.2, 46.4; BaP 96.7, 88.9 | Dermal permeabilities were increased in 2–3 rings PAHs; Bap metabolism was affected by the levels and duration of exposure and the age of skin. |
| Model (Dosing Vehicle) | PAHs | Units | Results | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Human breast skin (acetone and tetrahydrofuran) | BaP dihydrodiols and tetrols | Range (pmol/cm2) | Concentrations extracted from skin BaP(7,8)-dihydrodiol: 1.71–18.27; BaP(4,5)-dihydrodiol: 0.24–10.43; BaP(9,10)-dihydrodiol: 1.03–12.65; BaP(7,9,10/8) tetrol: 4.6–10230; BaP(7,9/8,10) tetrol: 16.5–2017.7; BaP(7/8,9,10) tetrol: 2.1–630.2; BaP(7,10/8,9) tetrol: 29–1015.8 | Observed interindividual variations in the stereoselective metabolism of BaP, which will conditionate the individual susceptibility to PAH-induced skin carcinogenesis. | [72] |
| Male C3H/HeJ mice (acetone and cyclohexane) | BaP | 50th survival (days) Number of death individuals (%) Counts (dimensionless) | Treatment with 0.01% BaP 50th survival: 464; Number of death individuals: 100; Total papilloma/group: 1; Total carcinoma/group: 28; Number of tumors: 27 Treatment with 0.001% BaP: 50th survival: 732; Number of death individuals: 50; Total papilloma/group: 2; Total carcinoma/group: 3; Number of tumors: 5 Treatment with 0.0001% BaP 50th survival: 727; Number of death individuals: 50; Total papilloma/group: 0; Total carcinoma/group: 0; Number of tumors: 0 | Groups treated with BaP at 0.01% had such a strong response due to the BaP alone that the sensitivity for assessing cocarcinogenic activity was limited. | [71] |
| Female FVB/N inbred mice (toluene and 5% DMSO) | BaP, DB(a,l)P | Mean ± SD (adducts/108 nucleotides) | Total DNA adducts BaP: 141 ± 37 DB(a,l)P: 45 ± 13 | Exposure produced primarily papillomas followed by squamous cell carcinoma and carcinoma in situ; BaP caused over three times the level of total DNA adducts; DB(a,l)P carcinogenicity was much higher than predicted. | [73] |
| Human cells—HaCaT cells (0.1% DMSO) | BaP, DB(a,l)P, Chry | EC50 (µmol/L) | Chry EC 50: 3.8; 2.0 DB(a,l)P EC 50: 0.035 | Chry caused strong cytotoxic effects in cell lines; BaP and DB(a,l)P up-regulated the levels of metabolites; CYP1A1 and CYP1B1 expression was significantly increased in some cell lines treated with BaP and Chry and the metabolites formed contributed to the observed metabolomic alterations. | [66] |
| Pig skin (30% propylene glycol/phosphate buffer, pH 7.4) | Naph, Fln, Pyr, Chry, BaA, BaP | (dimensionless) | Toxicological index of PAHs Ciclo-oxigenase-2 Naph: 0.01; Fln: 0.09; Pyr: 0.05; Chry: 0.60; BaA: 0.91; BaP: 0.97 Prostaglandin E2 Naph: 0.07; Fln: 0.34; Pyr: 0.15; Chry: 2.21; BaA: 5.61; BaP: 4.43 Chemokine (C-X-C motif) ligand Naph: 0.34; Fln: 0.81; Pyr: 0.31; Chry: 1.83; BaA: 4.00; BaP: 4.66 Interleukin-8 Naph: 0.08; Fln: 0.19; Pyr: 0.07; Chry: 0.47; BaA: 1.08; BaP: 1.84 | BaA and BaP were the compounds revealing the great skin inflammation and barrier function damage. | [13] |
| CALUX Bioassay (0.8% DMSO) | PAH metabolites | EC20, EC50 (mol/L) REP EC20, EC50 (dimensionless) | Concentrations measured on the assay 1-Hydroxynaphthalene: EC20, EC50 > 2.40 × 10−4 2-Hydroxynaphthalene: EC20: 3.54 × 10−5; EC50: 7.50 × 10−5 REP EC20: 2.99 × 10−5; REP EC50: 6.08 × 10−5 1-Hydroxyphenanthrene: EC20, EC50 > 1.00 × 10−5 2-Hydroxyphenanthrene: EC20: 3.93 × 10−5; EC50: 8.11 × 10−5 REP EC20: 2.69 × 10−5; REP EC50: 5.63 × 10−5 3-Hydroxyphenanthrene: EC20, EC50 > 1.00 × 10−5 4-Hydroxyphenanthrene: EC20: 1.83 × 10−6; EC50: 7.75 × 10−6 REP EC20: 5.78 × 10−4; REP EC50: 5.89 × 10−4 9-Hydroxyphenanthrene: EC20, EC50 > 1.00 × 10−5 2-Hydroxyfluorene: EC20, EC50 > 8.00 × 10−4 3-Hydroxyfluorene: EC20: 2.38 × 10−6; EC50: 8.43 × 10−6 REP EC20: 4.44 × 10−4; REP EC50: 5.41 × 10−4 4-Hydroxyfluorene: EC20, EC50 > 3.00 × 10−5 1-Hydroxypyrene: EC20: 1.09 × 10−4; EC50 > 1.00 × 10−4 REP EC20: 9.70 × 10−6 3-Hydroxychrysene: EC20: 2.46 × 10−7; EC50: 1.03 × 10−6 REP EC20: 4.30 × 10−3; REP EC50: 4.43 × 10−3 6-Hydroxychrysene: EC20: 3.06 × 10−6; EC50: 1.34 × 10−5 REP EC20: 3.45 × 10−4; REP EC50: 3.40 × 10−4 3-Hydroxybenzo(a)pyrene: EC20, EC50 > 8.00 × 10−6; BaP: EC20: 1.06 × 10−9; EC50: 4.56 × 10−9 REP EC20, REP EC50: 1.00 | Increased bioassay response with extracts from post-fire neck and calf wipe samples; Correlation between the bioassay response and urinary levels of PAH metabolites. | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, G.; Teixeira, J.; Delerue-Matos, C.; Sarmento, B.; Morais, S.; Wang, X.; Rodrigues, F.; Oliveira, M. Exposure to PAHs during Firefighting Activities: A Review on Skin Levels, In Vitro/In Vivo Bioavailability, and Health Risks. Int. J. Environ. Res. Public Health 2022, 19, 12677. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191912677
Sousa G, Teixeira J, Delerue-Matos C, Sarmento B, Morais S, Wang X, Rodrigues F, Oliveira M. Exposure to PAHs during Firefighting Activities: A Review on Skin Levels, In Vitro/In Vivo Bioavailability, and Health Risks. International Journal of Environmental Research and Public Health. 2022; 19(19):12677. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191912677
Chicago/Turabian StyleSousa, Gabriel, Joana Teixeira, Cristina Delerue-Matos, Bruno Sarmento, Simone Morais, Xianyu Wang, Francisca Rodrigues, and Marta Oliveira. 2022. "Exposure to PAHs during Firefighting Activities: A Review on Skin Levels, In Vitro/In Vivo Bioavailability, and Health Risks" International Journal of Environmental Research and Public Health 19, no. 19: 12677. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191912677