Effect of Electrostatic Field Strength on Bioelectrochemical Nitrogen Removal from Nitrogen-Rich Wastewater
Abstract
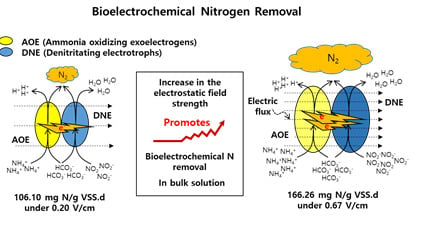
:1. Introduction
2. Materials and Methods
2.1. Bioelectrochemical Reactor Set Up and Operation
2.2. Analytical Techniques and Calculation
2.3. Bacterial Community Analysis
3. Results and Discussion
3.1. Bioelectrochemical Removal of Ammonium and Nitrite
3.2. Nitrite and Alkalinity Requirements
3.3. Electrochemical Properties in the Bulk Solution
3.4. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AOE | Ammonium oxidizing exoelectrogens |
| DNE | Denitritating electrotrophs |
| BENR | Bioelectrochemical nitrogen removal reactors |
| BNR | Biological nitrogen removal |
| CV | Cyclic voltammogram |
| DIET | Direct interspecies electron transfer |
| EIS | Electrochemical impedance spectroscopy |
| VSS | Volatile suspended solids |
References
- Ahn, Y.H. Sustainable nitrogen elimination biotechnologies: A review. Process Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Cao, S.; Wang, S.; Wu, C. Advanced nitrogen removal from wastewater by combining anammox with partial denitrification. Bioresour. Technol. 2015, 179, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; Ibrahim, A.; Assabri, A.M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technol. 2014, 258, 20–31. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2015, 200, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.S.; Joseph, K. Nitrogen management in landfill leachate: Application of SHARON, ANAMMOX and combined SHARON–ANAMMOX process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Mohan, S.V.; Lens, P.N.L. Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresour. Technol. 2016, 215, 173–185. [Google Scholar] [CrossRef]
- Komorowska-Kaufman, M.; Majcherek, H.; Klaczyński, E. Factors affecting the biological nitrogen removal from wastewater. Process Biochem. 2006, 41, 1015–1021. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, P.; Shen, L.; Ding, S.; Mahmood, Q. Kinetic characteristics and microbial community of Anammox-EGSB reactor. J. Hazard. Mater. 2011, 190, 28–35. [Google Scholar] [CrossRef]
- Jin, R.C.; Ma, C.; Yu, J.J. Performance of an Anammox UASB reactor at high load and low ambient temperature. Chem. Eng. J. 2013, 232, 17–25. [Google Scholar] [CrossRef]
- He, Z.; Kan, J.; Wang, Y.; Huang, Y.; Mansfeld, F.; Nealson, K.H. Electricity production coupled to ammonium in a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Feng, H.; Wang, M.; Li, N.; Cong, Y.; Shen, D. How to ascertain the importance of autotrophic denitrification process in a bioelectrochemical system. Bioresour. Technol. 2013, 146, 525–529. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Lee, S.H.; Park, H.D.; Min, B. Bacterial communities in a bioelectrochemical denitrification system: The effects of supplemental electron acceptors. Water Res. 2014, 51, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Fan, B.; Zhu, S.; Zheng, Y. Anaerobic ammonium oxidation with an anode as the electron acceptor. Environ. Microbiol. Rep. 2014, 6, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Zhan, G.; Li, D.; Tao, Y.; Zhu, X.; Zhang, L.; Wang, Y.; He, X. Ammonia as carbon-free substrate for hydrogen production in bioelectrochemical systems. Int. J. Hydrogen Energy 2014, 39, 11845–11859. [Google Scholar] [CrossRef]
- Mook, W.T.; Aroua, M.K.T.; Chakrabarti, M.H.; Noor, I.M.; Irfan, M.F.; Low, C.T.J. A review on the effect of bio-electrodes on denitrification and organic matter removal processes in bio-electrochemical systems. J. Ind. Eng. Chem. 2013, 19, 1–13. [Google Scholar] [CrossRef]
- Joicy, A.; Song, Y.C.; Lee, C.Y. Electroactive microorganisms enriched from activated sludge remove nitrogen in bioelectrochemical reactor. J. Environ. Manag. 2019, 233, 249–257. [Google Scholar] [CrossRef]
- Song, Y.C.; Joicy, A.; Jang, S.H. Direct interspecies electron transfer in bulk solution significantly contributes to bioelectrochemical nitrogen removal. Int. J. Hydrogen Energy 2019, 44, 2180–2190. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1666–1671. [Google Scholar] [CrossRef]
- Mousavi, S.; Ibrahim, S.; Aroua, M.K.; Ghafari, S. Development of nitrate elimination by autohydrogenotrophic bacteria in bio-electrochemical reactors—A review. Biochem. Eng. J. 2012, 67, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Polarized electrodes enhances biological direct interspecies electron transfer for methane production in upflow anaerobic bioelectrochemical reactor. Chemosphere 2018, 204, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Joicy, A.; Song, Y.C.; Yu, H.; Chae, K.J. Nitrite and nitrate as electron acceptors for bioelectrochemical ammonium oxidation under electrostatic field. J. Environ. Manag. 2019, 250. [Google Scholar] [CrossRef]
- Song, Y.C.; Kim, D.S.; Woo, J.H.; Subha, B.; Jang, S.H.; Sivakumar, S. Effect of surface modification of anode with surfactant on the performance of microbial fuel cell. Int. J. Energy Res. 2015, 39, 860–868. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Ahn, Y. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor. Bioresour. Technol. 2018, 259, 119–127. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Franson, M.A.H., Eds.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 10, 3968–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, G.; Bae, D.; Nickens, K.; O’Brien, T.J.; Patierno, S.R.; Ceryak, S. Polo-like kinase 1 enhances survival and mutagenesis after genotoxic stress in normal cells through cell cycle checkpoint bypass. Carcinogenesis 2010, 31, 785–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatkowska, A.B.; Paulsrud, B. The anammox process for nitrogen removal from wastewater–achievements and future challenges. Innsendte Artikler 2014, 02, 186–194. [Google Scholar]
- Zhang, Y.; Niu, Q.; Ma, H.; He, S.; Kubota, K.; Li, Y.Y. Long-term operation performance and variation of substrate tolerance ability in an anammox attached film expanded bed (AAFEB) reactor. Bioresour. Technol. 2016, 211, 31–40. [Google Scholar] [CrossRef]
- English, N.J.; Waldron, C.J. Perspectives on external electric fields in molecular simulation: Progress, prospects and challenges. Phys. Chem. Chem. Phys. 2015, 17, 12407. [Google Scholar] [CrossRef]
- Pansini, F.N.N.; De Souza, F.A.L.; Campos, C.T. Molecules Under External Electric Field: On the Changes in the Electronic Structure and Validity Limits of the Theoretical Predictions. J. Comput. Chem. 2018, 39, 1561–1567. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Xie, S.; Han, S.; Mei, L. Polaron tunneling in copolymers under external electric fields. Opt. Mater. 2003, 23, 479–483. [Google Scholar] [CrossRef]
- Lu, S.Z.; Li, X.Y.; Liu, J.F. Effect of external electric field on electron transfer in conjugated molecular wire. Chem. Phys. 2004, 297, 31–37. [Google Scholar] [CrossRef]
- Rivas, L.; Soares, C.M.; Baptista, A.M.; Simaan, J.; Paolo, R.E.D.; Murgid, D.H.; Hildebrandt, P. Electric-Field-Induced Redox Potential Shifts of Tetraheme Cytochromes c3 Immobilized on Self-Assembled Monolayers: Surface-Enhanced Resonance Raman Spectroscopy and Simulation Studies. Biophys. J. 2005, 88, 4188–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisseroth, K.P.; Braune, H. Thermodynamic equilibrium in strong electric fields and field chemistry consequences. J. Phys. 1977, 38, 1249–1255. [Google Scholar] [CrossRef]
- Xiang, L.; Tao, N.J. Reactions triggered electrically. Nature 2016, 531, 38–39. [Google Scholar] [CrossRef]
- Newman, D.K.; Kolter, R. A role for excreted quinones in extracellular electron transfer. Nature 2000, 405, 94–97. [Google Scholar] [CrossRef]
- Canstein, H.V.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of Flavins by Shewanella Species and Their Role in Extracellular Electron Transfer. Appl. Environ. Microbiol. 2008, 74, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Richter, K.; Schicklberger, M.; Gescher, J. Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl. Environ. Microbiol. 2011, 78, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Cheng, Y.Y.; Li, B.B.; Li, W.W.; Li, D.B.; Yu, H.Q. Electron acceptor dependence of electron shuttle secretion and extracellular electron transfer by Shewanella oneidensis MR-1. Bioresour. Technol. 2013, 136, 711–714. [Google Scholar] [CrossRef]
- Doyle, L.E.; Marsili, E. Methods for enrichment of novel electrochemically-active microorganisms. Bioresour. Technol. 2015, 195, 273–282. [Google Scholar] [CrossRef]
- Dubé, C.D.; Guiot, S.R. Direct Interspecies Electron Transfer in Anaerobic Digestion: A Review. Adv. Biochem. Eng. Biotechnol. 2015, 151, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.P.; Hui, W.; Wang, Q.; Roh, S.W.; Shi, X.Q.; Shi, J.H.; Quan, Z.X. Pseudomonas caeni sp. nov., a denitrifying bacterium isolated from the sludge of an anaerobic ammonium-oxidizing bioreactor. Int. J. Syst. Evol. Microbiol. 2009, 59, 2594–2598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heylen, K.; Vanparys, B.; Wittebolle, L.; Verstraete, W.; Boon, N.; Vos, P.D. Cultivation of denitrifying bacteria: Optimization of isolation conditions and diversity study. Appl. Environ. Microbiol. 2006, 72, 2637–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, A.; Tindall, B.J.; Bardin, V.; Blanchet, D.; Jeanthon, C. Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int. J. Syst. Evol. Microbiol. 2005, 55, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ren, Z.J.; Huang, C.; Liu, B.; Ren, N.; Xing, D. Multiple syntrophic interactions drive biohythane production from waste sludge in microbial electrolysis cells. Biotechnol. Biofuels 2016, 9, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.B.; Jiang, Z.; Chen, C.; Yuan, Y.; Gao, L.F.; Wang, H.F.; Cheng, J.; Li, W.J.; Wang, A.J. Thiopseudomonas denitrificans gen. nov., sp. nov., isolated from anaerobic activated sludge. J. Syst. Evol. Microbiol. 2015, 65, 225–229. [Google Scholar] [CrossRef] [Green Version]






| Contents | BENR20 | BENR33 | BENR67 | |
|---|---|---|---|---|
| Electrostatic field (V/cm) | 0.20 | 0.33 | 0.67 | |
| Specific NH4-N removal rate (mg/g VSS.d) | 59.6 | 64.0 | 81.5 | |
| NO2-N/NH4-N (mg/mg) | 0.80 ± 0.12 | 1.15 ± 0.13 | 1.04 ± 0.03 | |
| Alkalinity/NH4-N (mg/L as CaCO3/mg) | 1.42 ± 0.13 | 1.11 ± 0.06 | 1.41 ± 0.06 | |
| VSS (mg/L) | 920 ± 0.00 | 860 ± 9.57 | 890 ± 9.65 | |
| Biogas (N2/CH4) (mL) | 561.0/14.7 | 602.2/15.1 | 651.0/5.1 | |
| Specific total N removal rate (mg/g VSS.d) | 107.28 ± 3.58 | 137.60 ± 3.20 | 166.26 ± 3.26 | |
| Electron balance | N2 (%) | 73.8 | 85.5 | 93.5 |
| CH4 (%) | 5.2 | 5.7 | 5.9 | |
| Others (biomass, losses, %) | 21.0 | 8.8 | 0.6 | |
| BENR | Electrostatic Field (V/cm) | Redox | Ep (V) | Ip (mA) | Rs/Rct (Ω/Ω) |
|---|---|---|---|---|---|
| BENR20 | 0.20 | Oxidation Reduction | −0.22 −0.04 −0.54 | 0.58 0.45 0.72 | 5.86/3.88 |
| BENR33 | 0.33 | Oxidation Reduction | −0.24 −0.04 −0.55 | 0.81 0.47 0.75 | 6.12/4.35 |
| BENR67 | 0.67 | Oxidation Reduction | −0.20 −0.01 −0.49 | 0.80 0.51 0.90 | 4.72/4.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joicy, A.; Song, Y.-C.; Li, J.; Oh, S.-E.; Jang, S.-H.; Ahn, Y. Effect of Electrostatic Field Strength on Bioelectrochemical Nitrogen Removal from Nitrogen-Rich Wastewater. Energies 2020, 13, 3218. https://0-doi-org.brum.beds.ac.uk/10.3390/en13123218
Joicy A, Song Y-C, Li J, Oh S-E, Jang S-H, Ahn Y. Effect of Electrostatic Field Strength on Bioelectrochemical Nitrogen Removal from Nitrogen-Rich Wastewater. Energies. 2020; 13(12):3218. https://0-doi-org.brum.beds.ac.uk/10.3390/en13123218
Chicago/Turabian StyleJoicy, Anna, Young-Chae Song, Jun Li, Sang-Eun Oh, Seong-Ho Jang, and Yongtae Ahn. 2020. "Effect of Electrostatic Field Strength on Bioelectrochemical Nitrogen Removal from Nitrogen-Rich Wastewater" Energies 13, no. 12: 3218. https://0-doi-org.brum.beds.ac.uk/10.3390/en13123218