Biomass and Lipid Production Potential of an Indian Marine Algal Isolate Tetraselmis striata BBRR1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Algal Strains
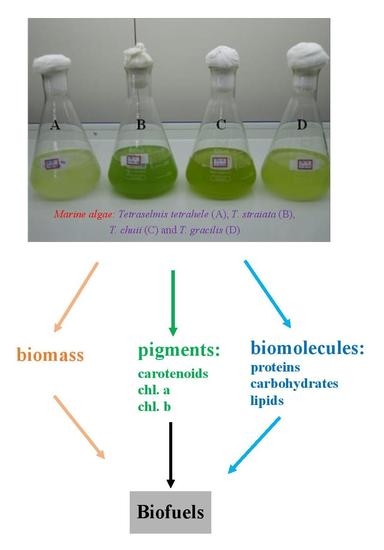
2.2. Laboratory Screening Studies
2.3. Molecular Characterisation
2.4. Acclimatisation of T. striata Butcher BBRR1 for Outdoor Cultivation
2.5. Mass Culture of T. striata BBRR1 in 10-m2 Open Raceway Pond
2.6. Biomass Harvest of T. striata BBRR1
2.7. Estimation of Biomass and Other Growth Parameters
2.8. Extraction of Algal Oil and Analysis of Fatty Acids
2.9. Acid Transesterification
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Tetraselmis Spp.
3.2. Screening Studies—Dry Biomass and Total Lipids in Tetraselmis spp.
3.3. Growth Performance of T. striata BBRR1 in 10-m2 Open Raceway Ponds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Vipin, C.; Aiyappan, A. Biological importance of marine algae an overview. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 222–227. [Google Scholar]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Mark, P.; McHenry, K.; de Boer, K.; Parisa, B. Biomass and Biofuels from Microalgae: Advances in Engineering and Biology; Springer: Cham, Germany, 2015. [Google Scholar]
- Florentinus, A.; Hamelinck, C.; Lint, S.D.; Iersel, S.V. Worldwide Potential of Aquatic Biomass; Ecofys: Utrecht, The Netherlands, 2008. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, T.; Hasan, M.; Md, C.; Aftabuddin, S.; Rahman, M.A.; Khan, M. Growth, Fatty Acid, and Lipid Composition of Marine Microalgae Skeletonemacostatum Available in Bangladesh Coast: Consideration as Biodiesel Feedstock. J. Mar. Biol. 2016, 8, 33–51. [Google Scholar]
- Griffiths, M.J.; Harrison, S.T. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Rodolfi, L.; ChiniZittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Iyengar, M.O.P.; Desikachary, T.V. Volvocales; Indian Council of Agricultural Research: New Delhi, India, 1981. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nanaHustedt and DetonulaconfervaceaCleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Moheimani, N.R. The Culture of Coccolithophore Algae for Carbon Dioxide Bioremediation. Ph.D. Thesis, Murdoch University, Perth, Australia, 2005. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.S.; Farrand, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 263–275. [Google Scholar]
- Folch, J.; Lees, M.; Sloane, S.G.H. Asimplemethodfortheisolationand purificationof thetotallipids fromanimaltissues. J. Biol. Chem. 1957, 226, 49–57. [Google Scholar]
- Richards, E.; Reichardt, M.; Rogers, S. Preparation of genomic DNA from plant tissue. Curr. Prot. Mol. Biol. 1994, 27, 2–3. [Google Scholar] [CrossRef]
- Liu, K.H.; Yeh, Y.L.; Shen, W.C. Fast preparation of fungal DNA for PCR screening. J. Microbiol. Meth. 2011, 85, 170–172. [Google Scholar] [CrossRef]
- Kawabata, Y.; Nakahara, H.; Katayama, Y.; Ishida, N. The phytoplankton of some saline lakes in Central Asia. Int. J. Salt Lake Res. 1997, 6, 5–16. [Google Scholar] [CrossRef]
- López-González, P.J.; Guerrero, F.; Carmen, C.M. Seasonal fluctuations in the plankton community in a hypersaline temporary lake (Honda, southern Spain). Inter. J. Salt Lake Res. 1998, 6, 353–371. [Google Scholar] [CrossRef]
- Huerlimann, R.; De Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef]
- Arkronrat, W.; Deemark, P.; Oniam, V. Growth performance and proximate composition of mixed cultures of marine microalgae (Nannochloropsis sp. and Tetraselmis sp.) with monocultures. Songklanakarin. J. Sci. Technol. 2016, 38, 326–345. [Google Scholar]
- Metzger, P.; Largeau, C. Botryococcusbraunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef]
- Fon-Sing, S.; Borowitzka, M.A. Isolation and screening of euryhaline Tetraselmis spp. Suitable for large-scale outdoor culture in hypersaline media for biofuels. J. Appl. Phycol. 2015, 28, 1–14. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Wu, W.T. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Tremblay, R.; Demers, E.; Roussy, M. Variation of lipid class and fatty acid composition of Chaetocerosmuelleri and Isochrysis sp. grown in a semicontinuous system. Aquaculture 2003, 221, 393–406. [Google Scholar] [CrossRef]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Zakwan, A. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmusbijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.L. Other micro-algae. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 135–150. [Google Scholar]





| Strains | Volumetric Biomass Productivity (g L−1 d−1) | Lipid Productivity (mg L−1 d−1) | Lipid Content (%) | CO2 Fixed (g L−1 d−1) |
|---|---|---|---|---|
| T. chuii | 0.023 ± 0.003 | 5.33 ± 0.005 | 31.46 ± 1.24 | 0.042 ± 0.012 |
| T. gracilis | 0.024 ± 0.004 | 5.12 ± 0.004 | 27.88 ± 1.12 | 0.042 ± 0.013 |
| T. striata | 0.025 ± 0.004 | 7.21 ± 0.005 | 33.60 ± 1.25 | 0.046 ± 0.025 |
| T. tetrathele | 0.021 ± 0.005 | 5.22 ± 0.004 | 28.48 ± 1.05 | 0.038 ± 0.017 |
| Growth Media | Volumetric Biomass Productivity (g L−1d−1) | Specific Growth Rate (Day−1) | Div. d−1 | Gen’ t (d) |
|---|---|---|---|---|
| f/2 (1×) | 0.022 ± 0.005 | 0.39 ± 0.07 | 0.56 ± 0.04 | 1.79 ± 0.08 |
| f (2×) | 0.027 ± 0.004 | 0.41 ± 0.06 | 0.59 ± 0.03 | 1.69 ± 0.05 |
| 5f (5×) | 0.019 ± 0.006 | 0.16 ± 0.03 | 0.24 ± 0.07 | 4.25 ± 0.03 |
| 10f (10×) | 0.003 ± 0.003 | 0.16 ± 0.04 | 0.23 ± 0.05 | 4.31 ± 0.05 |
| Growth Medium | Volumetric Biomass Productivity (g L−1 d−1) | Specific Growth Rate (day–1) | Div. d−1 | Gen’ t (d) | Lipid Productivity (mg L−1 d−1) |
|---|---|---|---|---|---|
| [A] Modified CFTRI | 0.81 ± 0.04 | 0.38 ± 0.06 | 0.55 ± 0.03 | 1.81 ± 0.07 | 8.41 ± 0.013 |
| [B] Modified CFTRI ABRR1 | 0.71 ± 0.08 | 0.60 ± 0.08 | 0.86 ± 0.05 | 1.16 ± 0.05 | 13.44 ± 0.015 |
| S.No | Culture Conditions | Specific Growth Rate (Day–1) | Div. d−1 | Gen’ t (d) | Volumetric Biomass Productivity (g L−1 d−1) | Areal Biomass Productivity (g m−2 d−1) | Lipid Content (%) |
|---|---|---|---|---|---|---|---|
| 1 | f/2-medium under in-vitro study | 0.39 ± 0.07 | 0.56 ± 0.04 | 1.79 ± 0.08 | 0.022 ± 0.02 | - | 25.07 ± 0.24 |
| 2 | f-medium under in-vitro study | 0.41 ± 0.06 | 0.59 ± 0.03 | 1.68 ± 0.08 | 0.027 ± 0.05 | - | 19.42 ± 0.80 |
| 3 | Modified CFTRI ABRR1 medium under in-vitro study | 0.60 ± 0.08 | 0.86 ± 0.05 | 1.16 ± 0.05 | 0.032 ± 0.04 | - | 18.76 ± 1.30 |
| 4 | 10-m2 open race way pond f-medium | 0.24 ± 0.06 | 0.35 ± 0.03 | 2.88 ± 0.04 | 0.055 ± 0.05 | 8.25 ± 0.06 | 16.50 ± 1.42 |
| 5 | 10-m2 open race way Modified CFTRI ABRR1medium | 0.45 ± 0.07 | 0.64 ± 0.05 | 1.55 ± 0.07 | 0.063 ± 0.08 | 9.45 ± 0.09 | 19.42 ± 0.98 |
| Fatty Acids | Wt. (%) |
|---|---|
| 7-Octadecene | 1.49 |
| Methyltetradecanoate | 2.63 |
| Pentadecanoic acid, methyl ester | 3.14 |
| Palmitoleic acid, methyl ester | 5.94 |
| Palmitic acid, methyl ester | 33.14 |
| Hexadecanoic acid, 14-methyl-, methyl ester | 2.82 |
| 11-Octadecenoic acid, methyl ester | 22.64 |
| Heptadecanoic acid, 16-methyl-, methyl ester | 21.94 |
| Cyclopropane octanoic acid 2-hexyl-methyl ester | 1.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boopathy, A.B.; Jayakumar, T.; Chinnasamy, S.; Rajaram, M.G.; Mohan, N.; Nagaraj, S.; Rengasamy, R.; Manubolu, M.; Sheu, J.-R.; Chang, C.-C. Biomass and Lipid Production Potential of an Indian Marine Algal Isolate Tetraselmis striata BBRR1. Energies 2020, 13, 341. https://0-doi-org.brum.beds.ac.uk/10.3390/en13020341
Boopathy AB, Jayakumar T, Chinnasamy S, Rajaram MG, Mohan N, Nagaraj S, Rengasamy R, Manubolu M, Sheu J-R, Chang C-C. Biomass and Lipid Production Potential of an Indian Marine Algal Isolate Tetraselmis striata BBRR1. Energies. 2020; 13(2):341. https://0-doi-org.brum.beds.ac.uk/10.3390/en13020341
Chicago/Turabian StyleBoopathy, Annakkili Baskara, Thanasekaran Jayakumar, Senthil Chinnasamy, Muthu Ganesan Rajaram, Natarajan Mohan, Subramani Nagaraj, Ramasamy Rengasamy, Manjunath Manubolu, Joen-Rong Sheu, and Chao-Chien Chang. 2020. "Biomass and Lipid Production Potential of an Indian Marine Algal Isolate Tetraselmis striata BBRR1" Energies 13, no. 2: 341. https://0-doi-org.brum.beds.ac.uk/10.3390/en13020341