Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch
Abstract
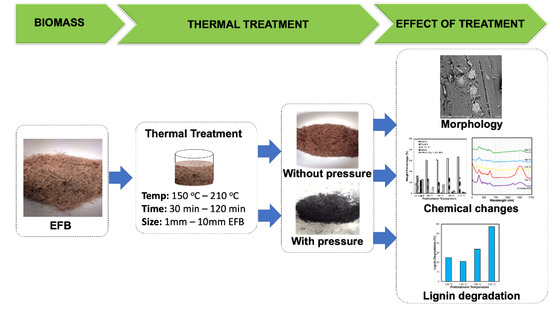
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Thermal Pretreatment of EFB
2.3. Characterization of EFB
2.4. Determination of Lignin Degradation
3. Results
3.1. Morphological External Surface
3.1.1. Physical Appearance Observation
3.1.2. SEM Observation
3.2. Chemical Compounds Analysis
3.3. Changes on Chemical Functional Groups
3.4. Effect of Thermal Treatment on Lignin Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nambiappan, B.; Ismail, A.; Hashim, N.; Ismail, N.; Shahari, D.N.; Idris, N.A.N.; Omar, N.; Salleh, K.M.; Hassan, N.A.M.; Kushairi, A. Malaysia: 100 Years of Resilient Palm Oil Economic Performance. J. Oil Palm Res. 2018, 30, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, V.; Abd-Talib, N.; Yong, T.-L.K. Lignin from oil palm empty fruit bunches (EFB) under subcritical phenol conditions as a precursor for carbon fiber production. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Ali, M.D.M.; Tamunaidu, P.; Aslan, A.K.H.N.; Morad, N.A.; Sugiura, N.; Goto, M.; Zhang, Z. Hydrothermal pre-treatment of oil palm empty fruit bunch into fermentable sugars. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012042. [Google Scholar]
- Roslan, R.; Zakaria, S.; Chia, C.H.; Boehm, R.; Laborie, M.-P. Physico-mechanical properties of resol phenolic adhesives derived from liquefaction of oil palm empty fruit bunch fibres, Industrial Crops and Products. Ind. Crop. Prod. 2014, 62, 119–124. [Google Scholar] [CrossRef]
- Krishnan, Y.; Bong, C.P.C.; Azman, N.F.; Zakaria, Z.; Othman, N.A.; Abdullah, N.; Ho, C.S.; Lee, C.T.; Hansen, S.B.; Hara, H. Co-composting of palm empty fruit bunch and palm oil mill effluent: Microbial diversity and potential mitigation of greenhouse gas emission. J. Clean. Prod. 2017, 146, 94–100. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Abnisa, F.; Daud, W.M.A.W.; Bakar, N.A.; Loh, S.K. A review of torrefaction of oil palm solid wastes for biofuel production. Energy Convers. Manag. 2017, 149, 101–120. [Google Scholar] [CrossRef]
- Palamae, S.; Dechatiwongse, P.; Choorit, W.; Chisti, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Rambo, M.K.D.; Schmidt, F.L.; Ferreira, M.M.C. Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 2015, 144, 696–703. [Google Scholar] [CrossRef]
- Chauhan, P.S. Role of various bacterial enzymes in complete depolymerization of lignin: A review. Biocatal. Agric. Biotechnol. 2020, 23, 101498. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 11636. [Google Scholar] [CrossRef] [Green Version]
- Sarip, H.; Hossain, M.S.; Azemi, M.; Allaf, K. A Review of the Thermal Pretreatment of Lignocellulosic Biomass towards Glucose Production: Autohydrolysis with DIC Technology. BioResources 2016, 11, 10625–10653. [Google Scholar]
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different pretreatment methods of lignocellulosic biomass for use in biofuel production. In Biomass for Bioenergy-Recent Trends and Future Challenges; IntechOpen: London, UK, 2019. [Google Scholar]
- Peng, F.; Peng, P.; Xu, F.; Sun, R.-C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Bu, D.; Xue, L.; Wang, Q.; Jiang, M. Degradation of lignin catalyzed by nanochannels solid acids in ionic liquid. Polym. Degrad. Stab. 2019, 166, 1–7. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Tiwikrama, A.H.; Wu, Y.-C.; Lee, M.-J. Alkali lignin degradation with aqueous ammonium-based ionic liquid solutions. J. Clean. Prod. 2020, 258, 120724. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Fontana, R.C.; Baudel, H.M.; Siqueira, F.G.d.; Jorge Rencoret, A.G.; Eugenio, L.I.d.; Prieto, A.; Martínez, M.J.; Martínez, Á.T.; Dillon, A.J.P.; et al. Lignin degradation and detoxification of eucalyptus wastes by on-site manufacturing fungal enzymes to enhance second-generation ethanol yield. Appl. Energy 2020, 26215, 114493. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Colpa, D.I.; Habib, M.H.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Bahrin, E.K.; Baharuddin, A.S.; Ibrahim, M.F.; Razak, M.N.A.; Sulaiman, A.; Abd-Aziz, S.; Hassan, M.A.; Shirai, Y.; Nishida, H. Physicochemical property changes and enzymatic hydrolysis enhancement of oil palm empty fruit bunches treated with superheated steam. BioResourse 2012, 7, 1784–1801. [Google Scholar]
- Shamsudin, S.; Shah, U.K.M.; Zainudin, H.; Abd Aziz, S.; Kamal, S.M.M.; Shirai, Y.; Hassan, M.A. Effect of steam pretreatment on oil palm empty fruit bunch for the production of sugars. Biomass Bioenergy 2012, 36, 280–288. [Google Scholar] [CrossRef]
- Chin, S.X.; Chia, C.H.; Zakaria, S.; Fang, Z.; Ahmad, S. Ball milling pretreatment and diluted acid hydrolysis of oil palm empty fruit bunch (EFB) fibres for the production of levulinic acid. J. Taiwan Inst. Chem. Eng. 2015, 52, 85–92. [Google Scholar] [CrossRef]
- Santanaraj, J.; Sajab, M.S.; Mohammad, A.W.; Harun, S.; Chia, C.H.; Zakaria, S.; Kaco, H. Enhanced delignification of oil palm empty fruit bunch fibers with in situ Fenton-oxidation. Bioresources 2017, 12, 5223–5235. [Google Scholar] [CrossRef] [Green Version]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2012, 1617, 1–16. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Cybulska, I.; Chaturvedi, T.; Thomsen, M.H. Lignocellulosic Thermochemical Pretreatment Processes; Bastidas-Oyanedel, J.R., Schmidt, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; p. 763. [Google Scholar]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Aita, G.A.; Kim, M. Pretreatment technologies for the conversion of lignocellulosic materials to bioethanol. In: Sustainability of the Sugar and Sugar–Ethanol Industries. Am. Chem. Soc. 2010, 117–145. [Google Scholar] [CrossRef]
- Isroi, C.A.; Panji, T.; Wibowo, N.A.; Syamsu, K. Bioplastic production from cellulose of oil palm empty fruit bunch. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012011. [Google Scholar] [CrossRef]
- Ariffin, H.; Hassan, M.A.; Kalsom, M.S.U.; Abdullah, N.; Shirai, Y. Effect of physical, chemical and thermal pretreatments on the enzymatic hydrolysis of oil palm empty fruit bunch. J. Trop. Agric. Food Sci. 2008, 36, 1–10. [Google Scholar]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef] [Green Version]
- Pituello, C.; Francioso, O.; Simonetti, G. Characterization of chemical–physical, structural and morphological properties of biochars from biowastes produced at different temperatures. J. Soils Sediments 2015, 15, 792–804. [Google Scholar] [CrossRef]
- Zulkefli, S.; Abdulmalek, E.; Rahman, M.B.A. Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk. Renew. Energy 2017, 107, 36–41. [Google Scholar] [CrossRef]
- Grube, M.; Lin, J.G.; Lee, P.H.; Kokorevicha, S. Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.; Ragauskas, A. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, H.; Luque, R. Advances on biomass pretreatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, J.-R.; Wang, Y.-T.; Zhu, M.-J. Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour. Technol. 2020, 301, 122756. [Google Scholar] [CrossRef]
- Bazargan, A.; Wang, Z.; Barford, J.P.; Saleem, J.; McKay, G. Optimization of the removal of lignin and silica from rice husks with alkaline peroxide. J. Clean. Prod. 2020, 260, 120848. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, A.; Balan, V.; Pareek, N.; Vivekanand, V. Biological treatment of lignocellulosic biomass by Chaetomium globosporum: Process derivation and improved biogas production. Int. J. Biol. Macromol. 2019, 128, 176–183. [Google Scholar] [CrossRef]
- Brahim, M.; Kantar, S.E.; Boussetta, N.; Grimi, N.; Brosse, N.; Vorobiev, E. Delignification of rapeseed straw using innovative chemo-physical pretreatments. Biomass Bioenergy 2016, 95, 92–98. [Google Scholar] [CrossRef]
- Yadav, M.; Paritosh, K.; Pareek, N.; Vivekanand, V. Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J. Clean. Prod. 2019, 213, 75–88. [Google Scholar] [CrossRef]
- Baksi, S.; Sarkar, U.; Saha, S.; Ball, A.K.; Kuniyal, J.C.; Wentzel, A.; Birgen, C.; Preisig, H.A.; Wittgens, B.; Markussen, S. Studies on delignification and inhibitory enzyme kinetics of alkaline peroxide pre-treated pine and deodar saw dust. Chem. Eng. Process. Process Intensif. 2019, 143, 107607. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Campanile, A.G.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Phutela, U.G.; Sahni, N.; Sooc, S.S. Fungal degradation of paddy straw for enhancing biogas production. Indian J. Sci. Technol. 2011, 4, 660–665. [Google Scholar] [CrossRef]






| Treatment Method | Operating Conditions | Biomass | Lignin Degradation (%) | Reference |
|---|---|---|---|---|
| Chemical treatment | NaOH pretreatment supplemented with H2O2 | Grass waste | 73.2 | [40] |
| Chemical treatment | 5.29% NaOH, 1% H2O2 at 20 °C | Rice husk | 59.85 | [41] |
| Physical treatment | Heating at 210 °C for 90 min at inert atmosphere | Oil palm EFB | 58 | This study |
| Biological treatment | Fungal strain (Chaetomium globosporum) at 36 °C for 31 days | Wheat straw | 45 | [42] |
| Chemo-physical treatment | 40 min treatment using high voltage electrical discharge treatment and NaOH (0.125–0.5 M) at 60–90 °C | Rapeseed straw | 42.3 | [43] |
| Biological treatment | Fungal strain (Pleurotus ostreatus) at 28 °C for 42 days | Pearl millet straw | 30 | [44] |
| Chemical treatment | 2% alkaline peroxide at 100 °C | Wood powders | 28.8 | [45] |
| Chemical treatment | ChCl:Urea (1:2) at 80 °C for 15 h | Corncob | 27.1 | [46] |
| Biological treatment | Fungal strain (Pleurotus ostreatus)at 28 °C for 42 days | Wheat straw | 21 | [44] |
| Biological treatment | Fungal strain (Coriolus versicolor)at 30 °C for 25 days | Paddy straw | 19 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, I.N.; Ongkudon, C.M.; Misson, M. Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch. Energies 2020, 13, 5966. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225966
Mohammad IN, Ongkudon CM, Misson M. Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch. Energies. 2020; 13(22):5966. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225966
Chicago/Turabian StyleMohammad, Intan Nazirah, Clarence M. Ongkudon, and Mailin Misson. 2020. "Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch" Energies 13, no. 22: 5966. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225966