A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
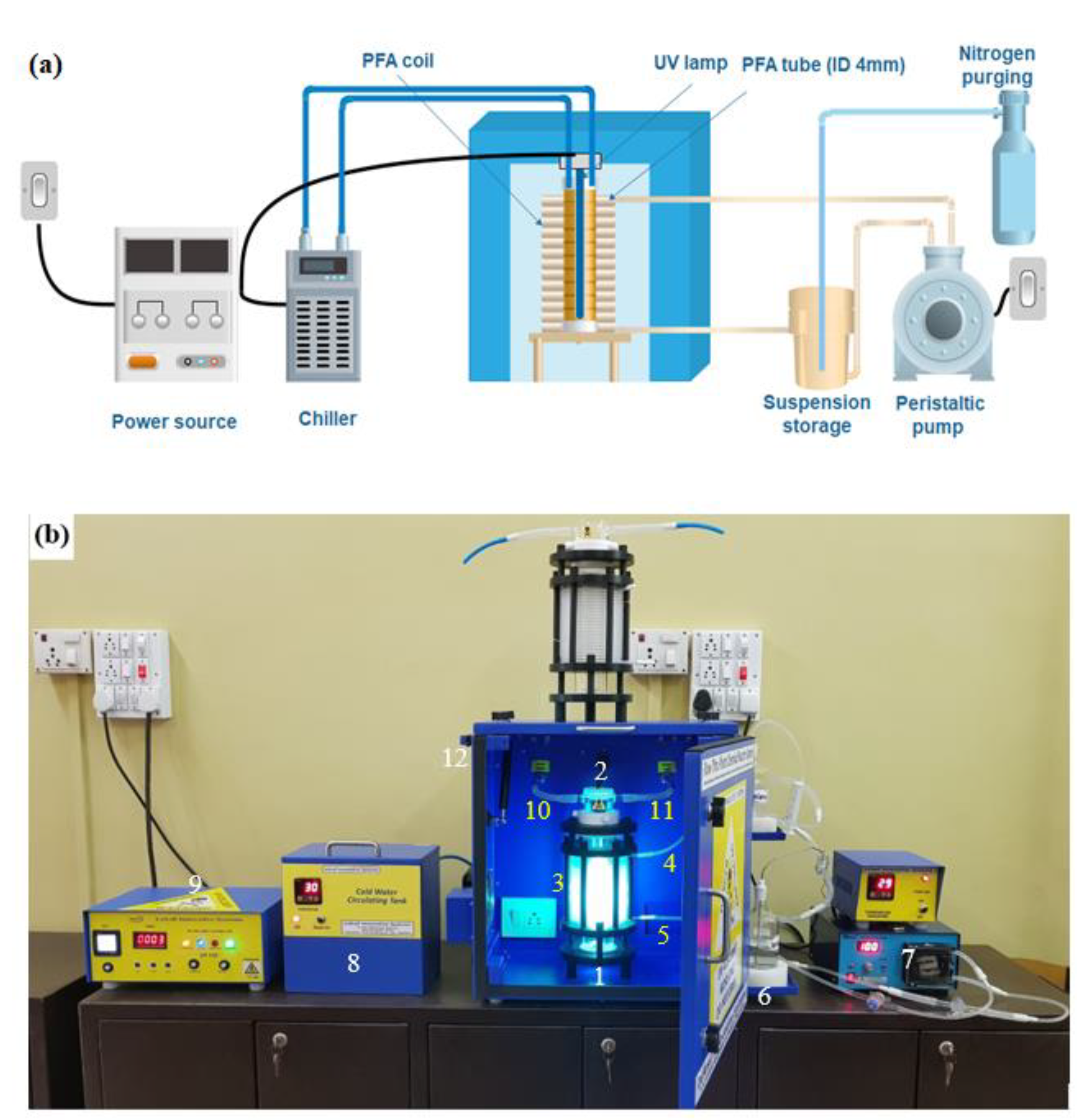
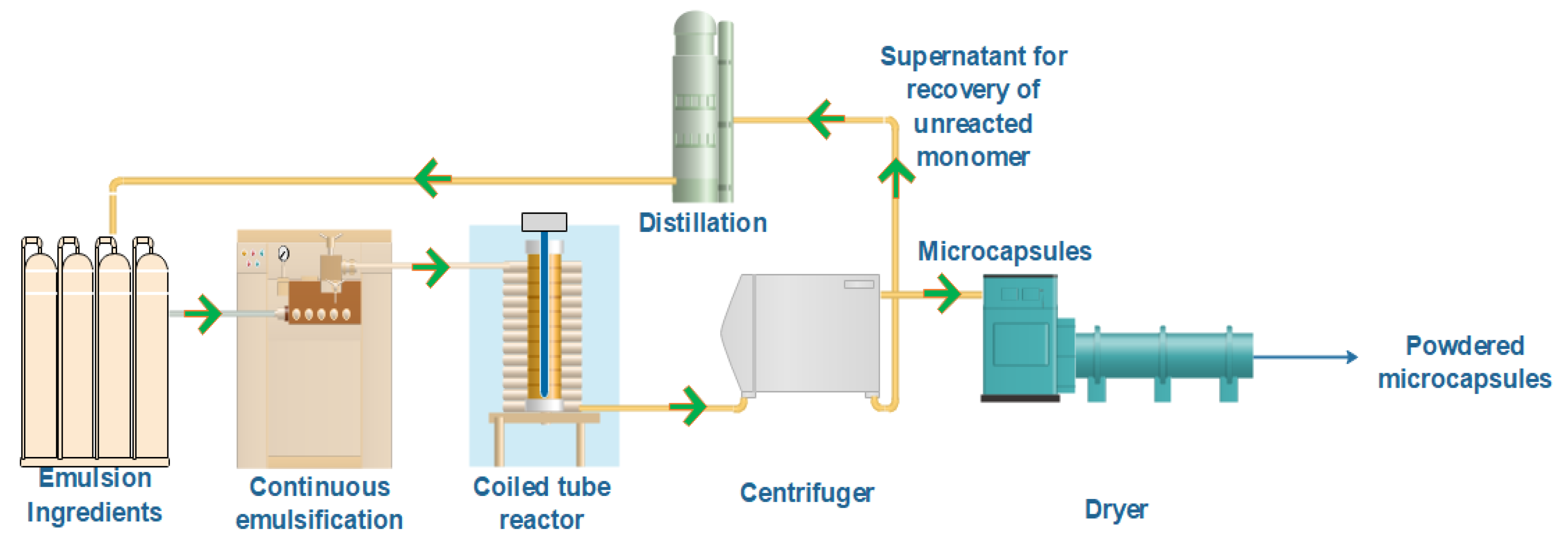
2.2.1. UV Equipment
2.2.2. Emulsion Preparation
2.2.3. Microencapsulation Procedure
2.3. Characterization of Microcapsules
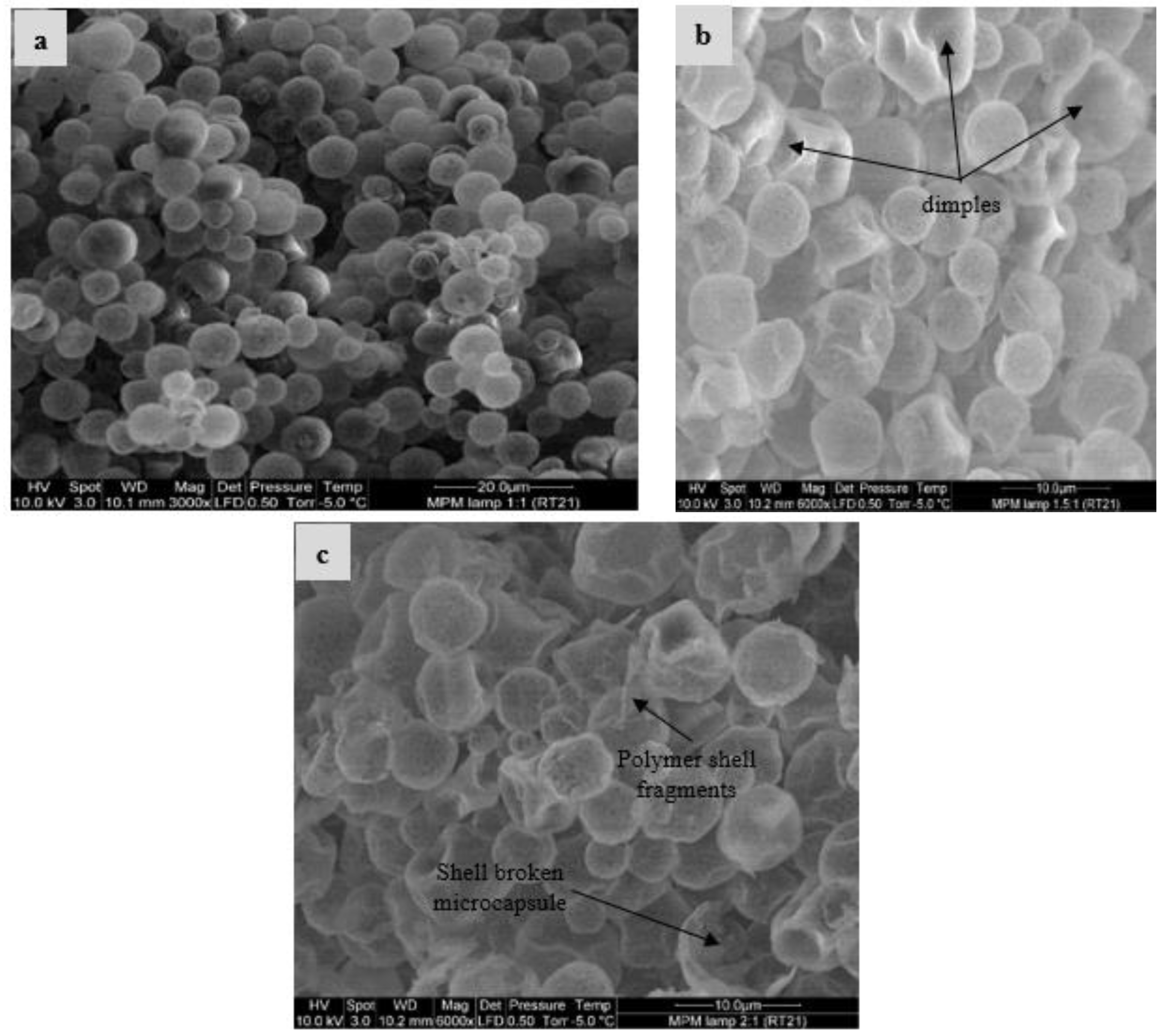
2.3.1. Scanning Electron Microscope
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermogravimetric Analysis
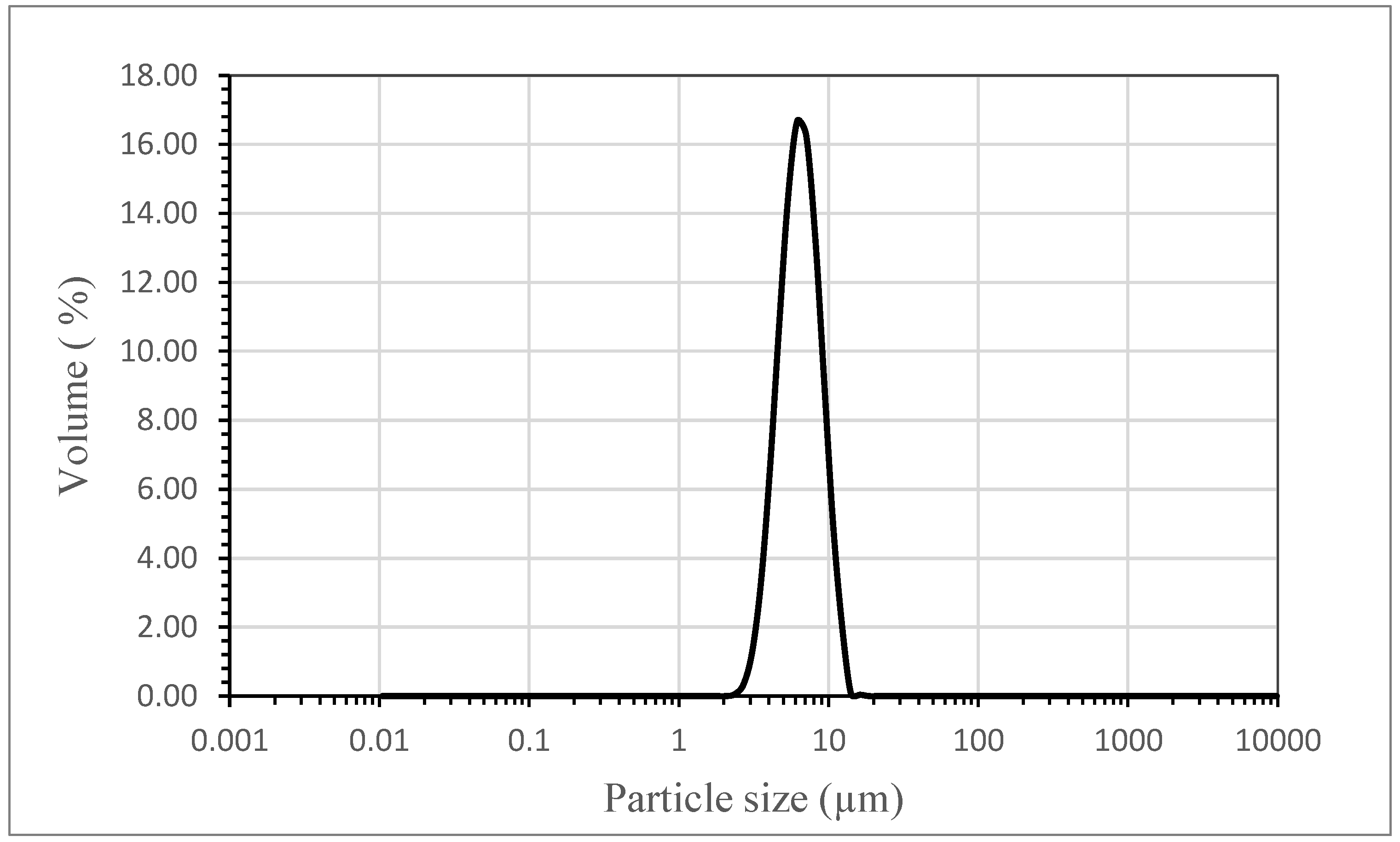
2.3.4. Particle Size and Particle Size Distribution (PSD)
2.3.5. Process Performance
3. Results and Discussions
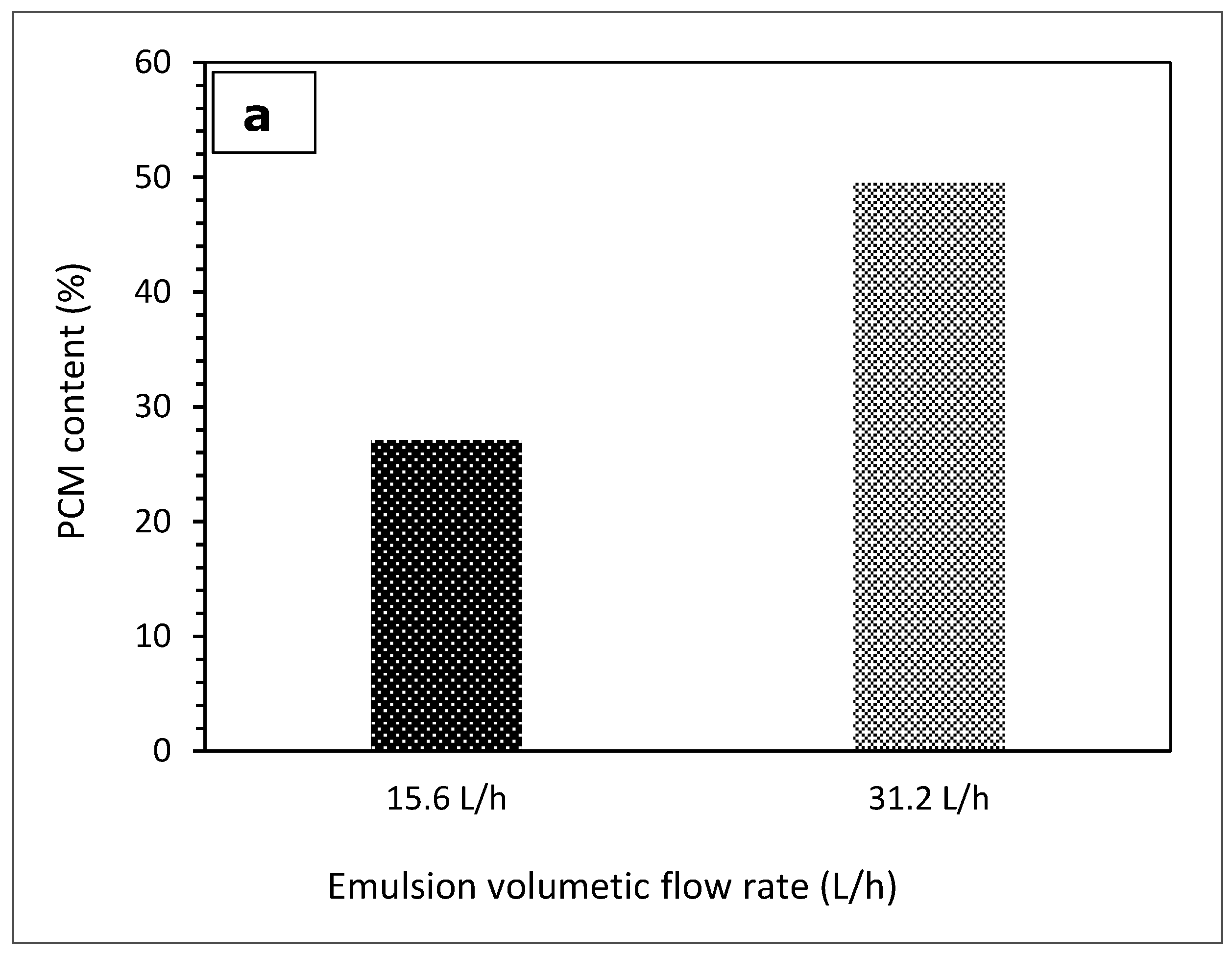
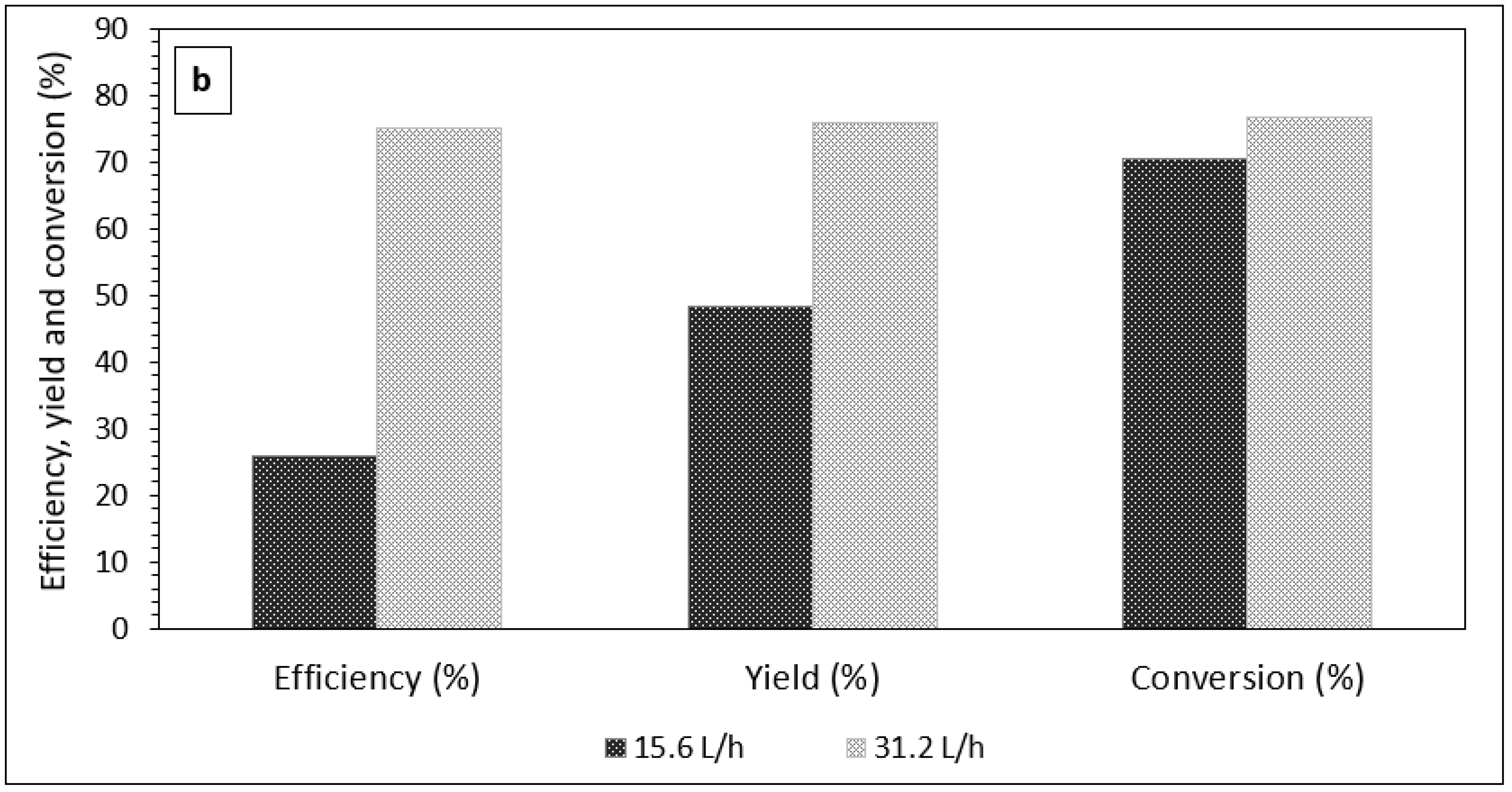
3.1. The Effect of Emulsion Volumetric Flow Rate on the Characteristics of PCM Microcapsules
3.2. The Effect of UV Treatment Time on the PCM Microcapsules Proprieties
3.3. The Effect of Different PCM-to-Monomer Mass Ratios on the PCM Microcapsules Proprieties
3.4. The Effect of UV Lamp Power on the PCM Microcapsule Characteristics
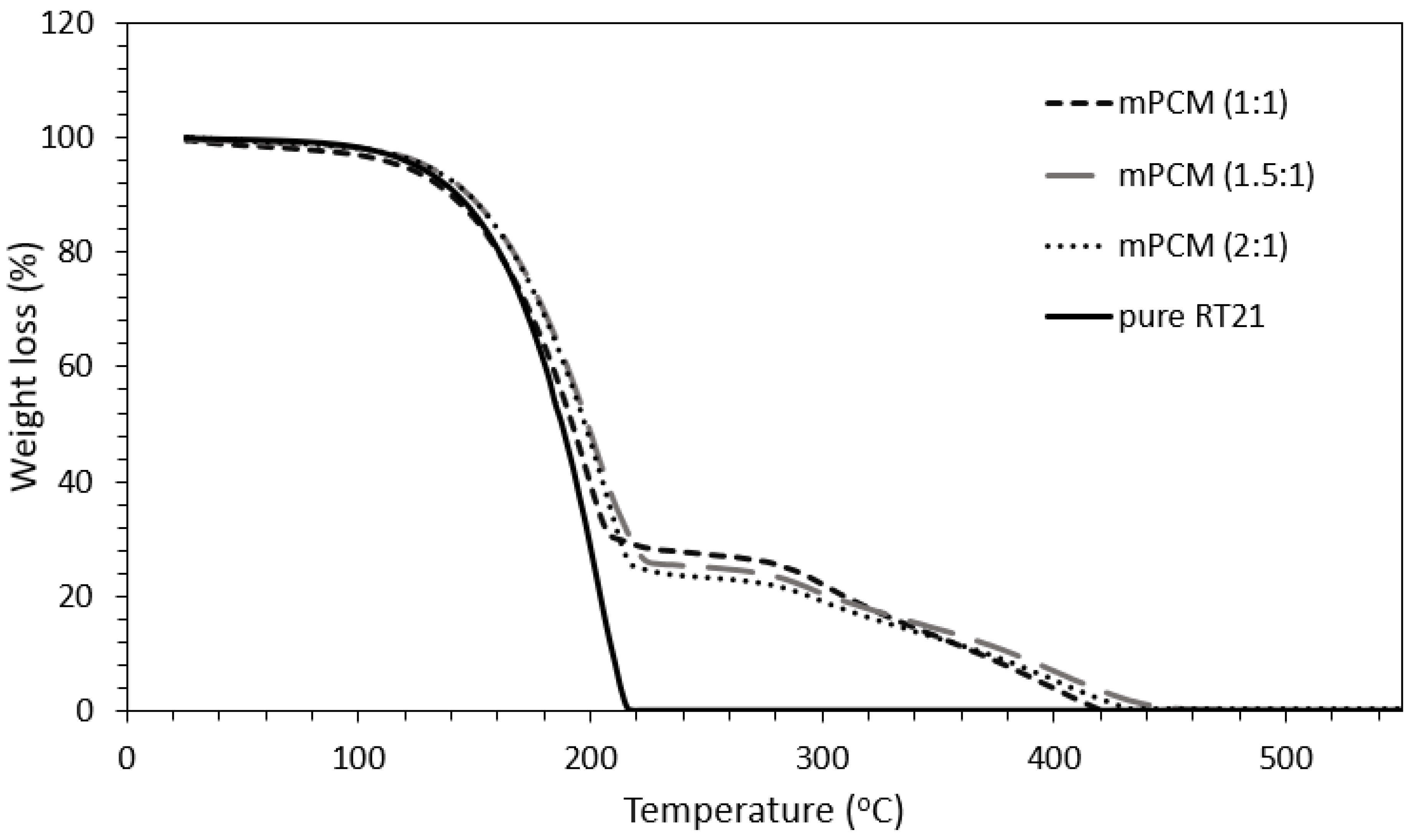
3.5. Thermal Stability
3.6. A Possibility of Continuous Microencapsulation Using Coiled Tube Reactor
- Cleaning process of PFA tubing;
- Integration of coiled tube reactor into a continuous process; and
- Mixing during UV treatment.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| PCM | phase change material | Tpm | peak melting temperature (°C) |
| mPCM | microencapsulated phase change material | Tpf | peak freezing temperature (°C) |
| TES | thermal energy storage | m | melting enthalpy (kJ/kg) |
| PFA | perfluoroalkoxy | freezing enthalpy (kJ/kg) | |
| PVA | polyvinyl alcohol | average enthalpies of melting and freezing (kJ/kg) | |
| EGDM | ethylene glycol dimethacrylate | ppm | part per million |
| MMA | methyl methacrylate | ID | internal diameter (mm) |
| PMMA | poly-methyl-methacrylate | µm | micrometer |
| RT | Rubitherm PCM | rpm | revolution per minute |
| SDS | sodium dodecyl sulfate | min | minute |
| Mw | molecular weight | W | watt |
| DSC | differential scanning calorimetry | L/h | liter/hour |
| TGA | thermo-gravimetric analysis | VF | volumetric flowrate (L/h) |
| SEM | scanning electron microscope | UV | ultraviolet |
| PSD | particle size distribution |
References
- Marin, P.; Saffari, M.; de Gracia, A.; Zhu, X.; Farid, M.; Cabeza, L.F.; Ushak, S. Energy savings due to the use of PCM for relocatable lightweight buildings passive heating and cooling in different weather conditions. Energy Build. 2016, 129, 274–283. [Google Scholar] [CrossRef] [Green Version]
- WGBC. Bringing Embodied Carbon Upfront: Coordinated Action for the Building and Construction Sector to Tackle Embodied Carbon; World Green Building Council: London, UK, 2019. [Google Scholar]
- Ikutegbe, C.A.; Farid, M.M. Application of phase change material foam composites in the built environment: A critical review. Renew. Sustain. Energy Rev. 2020, 131, 110008. [Google Scholar] [CrossRef]
- Schmerse, E.; Ikutegbe, C.A.; Auckaili, A.; Farid, M.M. Using PCM in Two Proposed Residential Buildings in Christchurch, New Zealand. Energies 2020, 13, 6025. [Google Scholar] [CrossRef]
- Kosny, J. PCM-Enhanced Building Components: An Application of Phase Change Materials in Building Envelopes and Internal Structures; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Yang, L.; Liu, J.; Qiao, Y.; Yan, D. Impacts of PCM Location and Thickness on Dynamic Thermal Characteristics of External Walls for Residential Buildings. In Modelling Environmental Dynamics; Springer: Singapore, 2020; pp. 127–135. [Google Scholar]
- Koschenz, M.; Lehmann, B. Development of a thermally activated ceiling panel with PCM for application in lightweight and retrofitted buildings. Energy Build. 2004, 36, 567–578. [Google Scholar] [CrossRef]
- Barzin, R.; Chen, J.J.; Young, B.R.; Farid, M. Application of PCM underfloor heating in combination with PCM wallboards for space heating using price based control system. Appl. Energy 2015, 148, 39–48. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.D.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Salaün, F. Phase Change Materials for Textile Application, Textile Industry and Environment; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Gin, B.; Farid, M. The use of PCM panels to improve storage condition of frozen food. J. Food Eng. 2010, 100, 372–376. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, A.; Shukla, M.; Chen, C.R. Development of thermal energy storage materials for biomedical applications. J. Med Eng. Technol. 2015, 39, 363–368. [Google Scholar] [CrossRef]
- Barzin, R.; Chen, J.J.; Young, B.R.; Farid, M.M. Application of weather forecast in conjunction with price-based method for PCM solar passive buildings—An experimental study. Appl. Energy 2015, 163, 9–18. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Young, B.; Farid, M. Cold energy storage in a packed bed of novel graphite/PCM composite spheres. Energy 2019, 171, 296–305. [Google Scholar] [CrossRef]
- Roberts, N.S.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Farid, M.M. Efficacy of using slurry of metal-coated microencapsulated PCM for cooling in a micro-channel heat exchanger. Appl. Therm. Eng. 2017, 122, 11–18. [Google Scholar] [CrossRef]
- Jaguemont, J.; Omar, N.; Van den Bossche, P.; Mierlo, J. Phase-change materials (PCM) for automotive applications: A review. Appl. Therm. Eng. 2018, 132, 308–320. [Google Scholar] [CrossRef]
- Al Shannaq, R.; Farid, M. Microencapsulation of phase change materials (PCMs) for thermal energy storage systems. In Advances in Thermal Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 247–284. [Google Scholar]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Tyagi, V.; Kaushik, S.; Tyagi, S.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Sánchez, L.; Sánchez, P.; de Lucas, A.; Carmona, M.; Rodríguez, J.F. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym. Sci. 2007, 285, 1377–1385. [Google Scholar] [CrossRef]
- Ma, S.; Song, G.; Li, W.; Fan, P.; Tang, G. UV irradiation-initiated MMA polymerization to prepare microcapsules containing phase change paraffin. Sol. Energy Mater. Sol. Cells 2010, 94, 1643–1647. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Romero, A.; Borreguero, A.M.; Carmona, M.; Sánchez, P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerisation. Chem. Eng. J. 2010, 157, 216–222. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Valverde, J.L.; Rodriguez, J.F.; Barber, A.; Cubillo, J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm®RT27 obtained by spray drying. Chem. Eng. J. 2011, 166, 384–390. [Google Scholar] [CrossRef]
- Aydın, A.A. In situ preparation and characterization of encapsulated high-chain fatty acid ester-based phase change material (PCM) in poly(urethane-urea) by using amino alcohol. Chem. Eng. J. 2013, 231, 477–483. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.; Al-Muhtaseb, S.; Kurdi, J. Emulsion stability and cross-linking of PMMA microcapsules containing phase change materials. Sol. Energy Mater. Sol. Cells 2015, 132, 311–318. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Dickinson, M.; Farid, M. Supercooling elimination of phase change materials (PCMs) microcapsules. Energy 2015, 87, 654–662. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Al-Shannaq, R.; Fernandez, A.I.; Farid, M.M. Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials 2015, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Y.; Zhao, L.; Shi, X.; Song, G.; Tang, G. A facile approach to synthesize microencapsulated phase change materials embedded with silver nanoparicle for both thermal energy storage and antimicrobial purpose. Energy 2018, 158, 1052–1059. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Chen, L.; Song, G.; Tang, G. Facile and low energy consumption synthesis of microencapsulated phase change materials with hybrid shell for thermal energy storage. J. Phys. Chem. Solids 2017, 111, 207–213. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Xia, T.D.; Zhang, T.; Feng, H.X. Fabrication and performances of microencapsulated paraffin composites with polymethylmethacrylate shell based on ultraviolet irradiation-initiated. Mater. Chem. Phys. 2012, 135, 181–187. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, M.; Zhang, Y.; Wang, Y. Microencapsulation of stearic acid with polymethylmethacrylate using iron (III) chloride as photo-initiator for thermal energy storage. Chin. J. Chem. Eng. 2017, 25, 1524–1532. [Google Scholar] [CrossRef]
- Farid, M.; Al-Shannaq, R.; Al-Muhtaseb, S.; Kurdi, J. Method for Low Temperature Microencapsulation of Phase Change Materials. U.S. Patent 10,913,882 B2, 9 February 2021. [Google Scholar]
- Shen, C.; Shang, M.; Zhang, H.; Su, Y. A UV—LEDs based photomicroreactor for mechanistic insights and kinetic studies in the norbornadiene photoisomerization. AIChE J. 2020, 66, e16841. [Google Scholar] [CrossRef]
- Dean, W., XVI. Note on the motion of fluid in a curved pipe. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1927, 4, 208–223. [Google Scholar] [CrossRef]
- Choudhary, R.; Bandla, S.; Watson, D.G.; Haddock, J.; Abughazaleh, A.; Bhattacharya, B. Performance of coiled tube ultraviolet reactors to inactivate Escherichia coli W1485 and Bacillus cereus endospores in raw cow milk and commercially processed skimmed cow milk. J. Food Eng. 2011, 107, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Gautam, D.; Umagiliyage, A.L.; Dhital, R.; Joshi, P.; Watson, D.G.; Fisher, D.J.; Choudhary, R. Nonthermal pasteurization of tender coconut water using a continuous flow coiled UV reactor. LWT 2017, 83, 127–131. [Google Scholar] [CrossRef]
- Koutchma, T.; Parisi, B.; Patazca, E. Validation of UV coiled tube reactor for fresh juices. J. Environ. Eng. Sci. 2007, 6, 319–328. [Google Scholar] [CrossRef]
- McDonough, J.; Murta, S.; Law, R.; Harvey, A. Oscillatory fluid motion unlocks plug flow operation in helical tube reactors at lower Reynolds numbers (Re ≤ 10). Chem. Eng. J. 2019, 358, 643–657. [Google Scholar] [CrossRef] [Green Version]
- Spasic, A.M. Chapter 1—Introduction. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 22, pp. 1–25. [Google Scholar]
- Abu-Bakar, A.S.; Moinuddin, K.A.M. Effects of Variation in Heating Rate, Sample Mass and Nitrogen Flow on Chemical Kinetics for Pyrolysis. In Proceedings of the 18th Australasian Fluid Mechanics Conference, Launceston, Australia, 3–7 December 2012. [Google Scholar]
- Erickson, K.L. Application of Low-Heating Rate TGA Results to Hazard Analyses Involving High-Heating Rates; Sandia National Lab.: Albuquerque, NM, USA, 2008. [Google Scholar]
- Alkan, C.; Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Convers. Manag. 2011, 52, 687–692. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, W.; Hao, S.; Liu, X.; Zhang, Z.; Shao, F. Ultraviolet light-initiated preparation of phase change material microcapsules and its infrared imaging effect on fabric. Pigment. Resin Technol. 2021, 50, 129–135. [Google Scholar] [CrossRef]


| PCM-to-Monomer Mass Ratio | Mass of Monomer (MMA) (g) | Mass of Cross-Linking Agent (EGDM) (g) | Mass of PCM (Paraffin RT21) (g) |
|---|---|---|---|
| 1:1 | 15.0 | 7.0 | 22.0 |
| 1.5:1 | 12.0 | 5.6 | 26.4 |
| 2:1 | 10.0 | 4.7 | 29.3 |
| Exp. No. | PCM-to-Monomer Mass Ratio | Volumetric Flow Rate (L/h) | Circulation Time (min) | Lamp Power (W) |
|---|---|---|---|---|
| 1 | 1:1 | 15.6 | 10 | 450 |
| 2 | 1:1 | 31.2 | 10 | 450 |
| 3 | 1:1 | 31.2 | 5 | 450 |
| 4 | 1:1 | 31.2 | 15 | 450 |
| 5 | 1.5:1 | 31.2 | 10 | 450 |
| 6 | 1.5:1 | 31.2 | 10 | 250 |
| 7 | 2:1 | 31.2 | 10 | 450 |
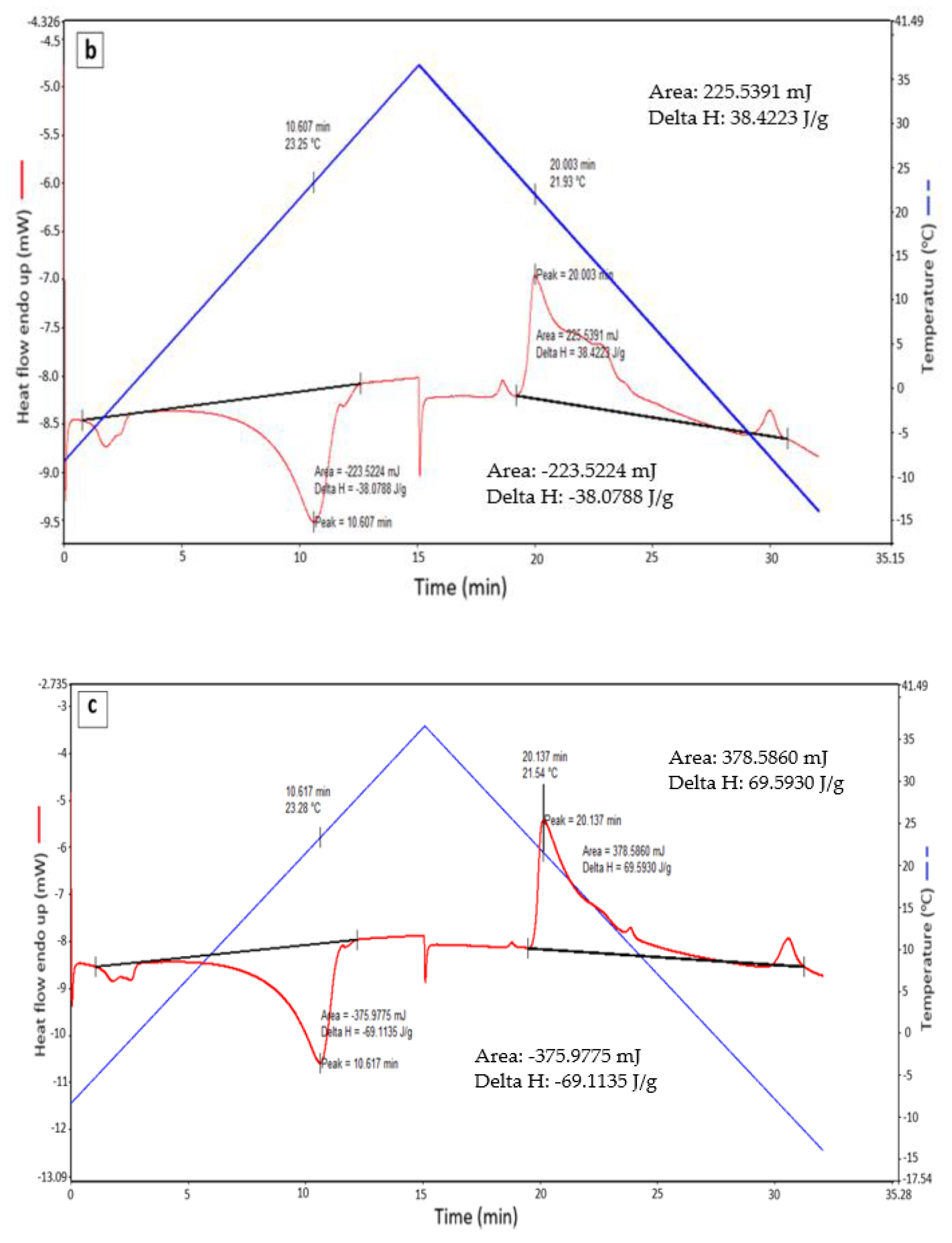
| Sample | VF (L/h) | Tpm (°C) | Hm (J/g) | Tpf (°C) | Hf (J/g) | Have (J/g) |
|---|---|---|---|---|---|---|
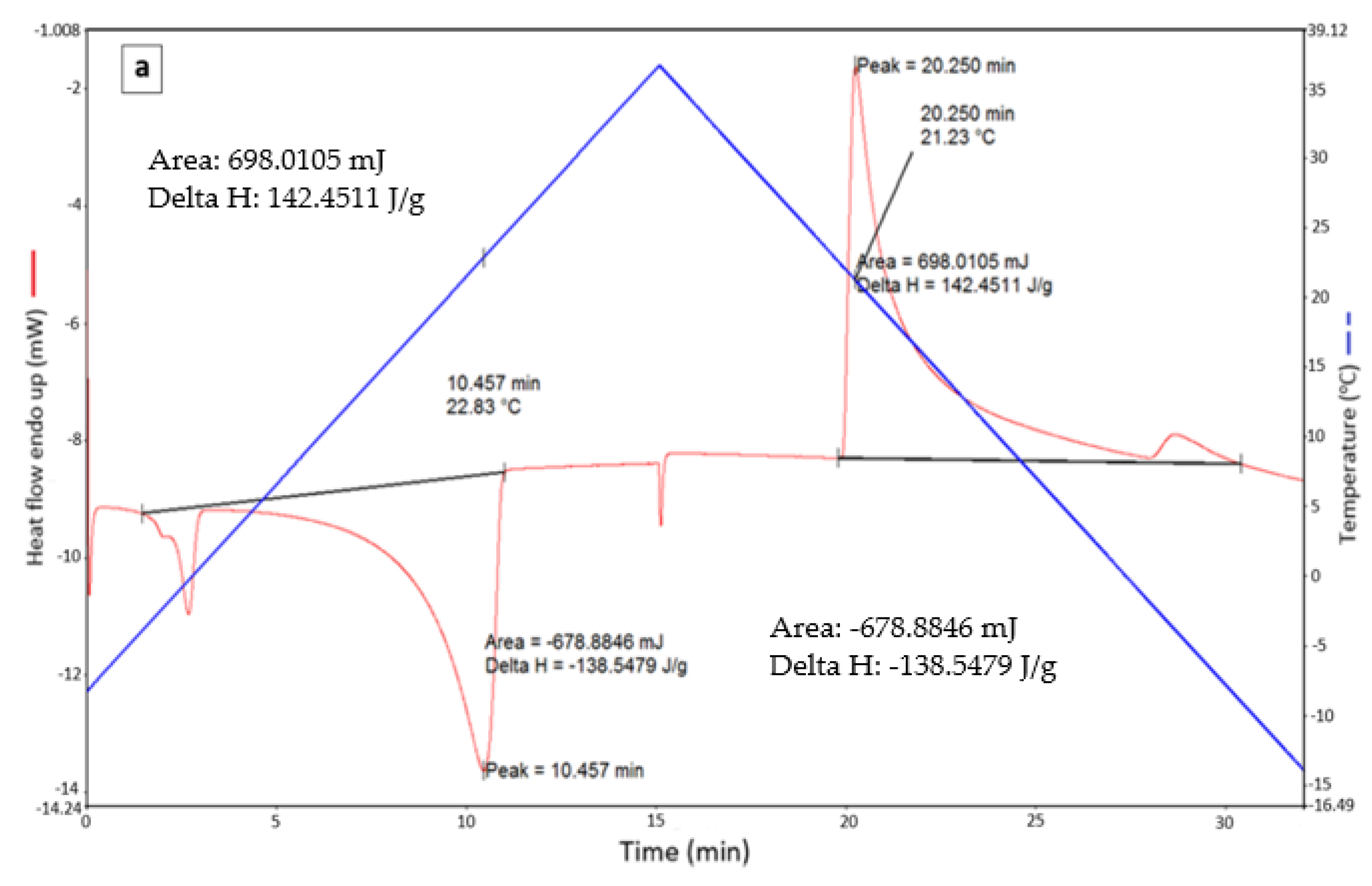
| Pure RT 21 | - | 22.83 | 138.55 | 21.23 | 142.45 | 140.5 |
| 1 | 15.6 | 23.25 | 38.08 | 21.93 | 38.42 | 38.25 |
| 2 | 31.2 | 23.28 | 69.11 | 21.54 | 69.59 | 69.35 |
| Reaction | Circulation Time (min) | Temp (°C) | Yield (%) | Efficiency (%) | Conversion (%) | % PCM Content in Microcapsules ɸ |
|---|---|---|---|---|---|---|
| Ultraviolet | 5 | 20–25 | 56.46 | 45.73 | 67.13 | 40.40 |
| 10 | 20–25 | 76.00 | 75.17 | 76.84 | 49.53 | |
| 15 | 20–25 | 70.21 | 74.93 | 65.41 | 53.77 |
| PCM-to-Monomer Mass Ratio | Yield (%) | Efficiency (%) | Conversion (%) | % PCM Content in Microcapsules ɸ |
|---|---|---|---|---|
| 1:1 | 76.00 | 75.17 | 76.84 | 49.53 |
| 1.5:1 | 59.22 | 59.84 | 58.28 | 60.97 |
| 2:1 | 55.56 | 48.13 | 70.47 | 57.83 |
| UV Lamp Power (W) | Yield (%) | Efficiency (%) | Conversion (%) | % PCM Content in Microcapsules ɸ |
|---|---|---|---|---|
| 250 | 77.40 | 85.80 | 64.78 | 66.54 |
| 450 | 59.22 | 59.84 | 58.27 | 60.97 |
| PCM/Shell | Tpm (°C) | Mt (min) | MT (°C) | UV Lamp Power (W) | PCM Content (%) | Microencapsulation Efficiency (%) | References |
|---|---|---|---|---|---|---|---|
| Paraffin/PMMA | 33.6 | 30 | 45 | 1000 | 61.2 | 61.2 | [25] |
| RT21/PMMA | 21 | 180 | 85 | - | 85.6 | - | [30] |
| Paraffin wax/PMMA | 56.3 | 30 | 60 | 250 | 66 | - | [35] |
| Eicosane/PMMA | 33.4 | 120 | 90 | - | 35 | - | [47] |
| Paraffin/PMMA | 55–60 | 30 | - | 1000 | 26.6 | - | [48] |
| RT21/PMMA | 21 | 10 | 25–30 | 250 | 49.5 | 85.8 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, J.A.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Ikutegbe, C.A.; Farid, M.M. A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor. Energies 2021, 14, 7867. https://0-doi-org.brum.beds.ac.uk/10.3390/en14237867
Ansari JA, Al-Shannaq R, Kurdi J, Al-Muhtaseb SA, Ikutegbe CA, Farid MM. A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor. Energies. 2021; 14(23):7867. https://0-doi-org.brum.beds.ac.uk/10.3390/en14237867
Chicago/Turabian StyleAnsari, Jawaad A., Refat Al-Shannaq, Jamal Kurdi, Shaheen A. Al-Muhtaseb, Charles A. Ikutegbe, and Mohammed M. Farid. 2021. "A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor" Energies 14, no. 23: 7867. https://0-doi-org.brum.beds.ac.uk/10.3390/en14237867








