Techno-Economic Analysis of Biodiesel Production from Microbial Oil Using Cardoon Stalks as Carbon Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process Simulation
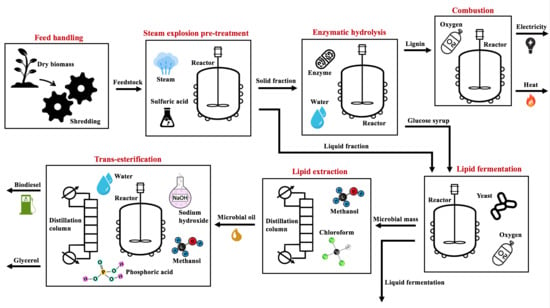
2.1.1. Feed Handling and Steam Explosion Pre-Treatment
2.1.2. Enzymatic Hydrolysis
2.1.3. Lipid Production
2.1.4. Lipid Extraction
2.1.5. Trans-Esterification
3. Results and Discussion
3.1. Simulation Results
3.1.1. Mass Balance
3.1.2. Energy Balance
3.1.3. Economic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felizardo, P.; Neiva Correia, M.J.; Raposo, I.; Mendes, J.F.; Berkemeier, R.; Bordado, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef]
- Pinzi, S.; Pilar dorado, M. Feedstocks for advanced biodiesel production. In Advances in Biodiesel Production; Elsevier: Amsterdam, The Netherlands, 2012; pp. 69–90. [Google Scholar]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Rahim, M.H.A.; Govindan, N.; Maniam, G.P. A first report on biodiesel production from Aglaia korthalsii seed oil using waste marine barnacle as a solid catalyst. Ind. Crops Prod. 2018, 125, 395–400. [Google Scholar] [CrossRef]
- Tamilalagan, A.; Singaram, J.; Rajamohan, S. Generation of biodiesel from industrial wastewater using oleaginous yeast: Performance and emission characteristics of microbial biodiesel and its blends on a compression injection diesel engine. Environ. Sci. Pollut. Res. 2019, 26, 11371–11386. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Papanikolaou, S. Biodiesel production from microbial oil. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2011; pp. 177–198. ISBN 9780857090492. [Google Scholar]
- Han, S.; Kim, G.-Y.; Han, J.-I. Biodiesel production from oleaginous yeast, Cryptococcus sp. by using banana peel as carbon source. Energy Rep. 2019, 5, 1077–1081. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A. Biological treatment of pulp and paper industry effluent by oleaginous yeast integrated with production of biodiesel as sustainable transportation fuel. J. Clean. Prod. 2017, 142, 2858–2864. [Google Scholar] [CrossRef]
- Deeba, F.; Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A.; Negi, Y.S. Amaranth seeds (Amaranthus palmeri L.) as novel feedstock for biodiesel production by oleaginous yeast. Environ. Sci. Pollut. Res. 2018, 25, 353–362. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Chatzifragkou, A.; Philippoussis, A.; Aggelis, G. Suitability of Low-Cost Sugars as Substrates for Lipid Production by the Fungus Thamnidium elegans. Energy Fuels 2010, 24, 4078–4086. [Google Scholar] [CrossRef]
- Leiva-Candia, D.E.; Pinzi, S.; Redel-Macías, M.D.; Koutinas, A.; Webb, C.; Dorado, M.P. The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar] [CrossRef]
- Gouda, M.K.; Omar, S.H.; Aouad, L.M. Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J. Microbiol. Biotechnol. 2008, 24, 1703–1711. [Google Scholar] [CrossRef]
- Chebbi, H.; Leiva-Candia, D.; Carmona-Cabello, M.; Jaouani, A.; Dorado, M.P. Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind. Crops Prod. 2019, 139, 111535. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.Y.; Lee, J.Y.; Ahn, C.Y.; Oh, H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Navarro-Juárez, R.; López-Martínez, J.; Campra-Madrid, P.; Rebolloso-Fuentes, M. Functional properties of the biomass of three microalgal species. J. Food Eng. 2004, 65, 511–517. [Google Scholar] [CrossRef]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of Biodiesel through Base-Catalyzed Transesterification of Safflower Oil Using an Optimized Protocol. Energy Fuels 2008, 22, 1306–1312. [Google Scholar] [CrossRef]
- Glisic, S.; Skala, D. The problems in design and detailed analyses of energy consumption for biodiesel synthesis at supercritical conditions. J. Supercrit. Fluids 2009, 49, 293–301. [Google Scholar] [CrossRef]
- Vlad, E.; Bildea, C.S.; Pleşu, V.; Marton, G.; Bozga, G. Process design of biodiesel production from rapeseed oil. Chem. Eng. Trans. 2010. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006. [Google Scholar] [CrossRef]
- Tasselli, G.; Filippucci, S.; Borsella, E.; D’Antonio, S.; Gelosia, M.; Cavalaglio, G.; Turchetti, B.; Sannino, C.; Onofri, A.; Mastrolitti, S.; et al. Yeast lipids from cardoon stalks, stranded driftwood and olive tree pruning residues as possible extra sources of oils for producing biofuels and biochemicals. Biotechnol. Biofuels 2018. [Google Scholar] [CrossRef]
- Kahr, H.; Pointner, M.; Krennhuber, K.; Wallner, B.; Jäger, A. Lipid production from diverse oleaginous yeasts from steam exploded corn cobs. Agron. Res. 2015, 13, 318–327. [Google Scholar]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, D.; Zhao, X. Pretreatment of lignocellulosic biomass for efficient enzymatic saccharification of cellulose. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–65. ISBN 9780128159361. [Google Scholar]
- Wiklund, A. The genus Cynara L. (Asteraceae-Cardueae). Bot. J. Linn. Soc. 1992, 109, 75–123. [Google Scholar] [CrossRef]
- Torres, C.M.; Ríos, S.D.; Torras, C.; Salvadó, J.; Mateo-Sanz, J.M.; Jiménez, L. Sustainability analysis of biodiesel production from Cynara Cardunculus crop. Fuel 2013, 111, 535–542. [Google Scholar] [CrossRef]
- Cravero, V.; Martin, E.; Crippa, I.; Anido, F.L.; García, S.M.; Cointry, E. Fresh biomass production and partitioning of aboveground growth in the three botanical varieties of Cynara cardunculus L. Ind. Crops Prod. 2012, 37, 253–258. [Google Scholar] [CrossRef]
- Ciancolini, A.; Alignan, M.; Pagnotta, M.A.; Vilarem, G.; Crinò, P. Selection of Italian cardoon genotypes as industrial crop for biomass and polyphenol production. Ind. Crops Prod. 2013, 51, 145–151. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Nassi o Di Nasso, N.; Bonari, E. Long-term evaluation of biomass production and quality of two cardoon (Cynara cardunculus L.) cultivars for energy use. Biomass Bioenergy 2009, 33, 810–816. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Biorefinery of Energy Crop Cardoon(Cynara cardunculus L.)-Hydrolytic Xylose Production as Entry Point to Complex Fractionation Scheme. J. Chem. Eng. Process Technol. 2011, 2, 8. [Google Scholar] [CrossRef]
- Galanopoulos, C.; Giuliano, A.; Barletta, D.; Zondervan, E. An integrated methodology for the economic and environmental assessment of a biorefinery supply chain. Chem. Eng. Res. Des. 2020, 160, 199–215. [Google Scholar] [CrossRef]
- Mabrouk, A.; Erdocia, X.; Alriols, M.G.; Labidi, J. Economic analysis of a biorefinery process for catechol production from lignin. J. Clean. Prod. 2018, 198, 133–142. [Google Scholar] [CrossRef]
- Aspen Technology Inc. Aspen Plus ® User Guide; Aspen Technology Inc.: Bedford, MA, USA, 2000. [Google Scholar]
- Facci, A.L.; Ubertini, S. Meta-heuristic optimization for a high-detail smart management of complex energy systems. Energy Convers. Manag. 2018, 160, 341–353. [Google Scholar] [CrossRef]
- Loreti, G.; Facci, A.L.; Baffo, I.; Ubertini, S. Combined heat, cooling, and power systems based on half effect absorption chillers and polymer electrolyte membrane fuel cells. Appl. Energy 2019, 235, 747–760. [Google Scholar] [CrossRef]
- García, A.; Alriols, M.G.; Llano-Ponte, R.; Labidi, J. Energy and economic assessment of soda and organosolv biorefinery processes. Biomass Bioenergy 2011, 35, 516–525. [Google Scholar] [CrossRef]
- Giuliano, A.; Poletto, M.; Barletta, D. Process Design of a Multi-Product Lignocellulosic Biorefinery. In Computer Aided Chemical Engineering; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 37, pp. 1313–1318. [Google Scholar]
- Meramo, S.; Ojeda, K.A.; Sánchez, E.L. Integrated Biorefinery from Corn Waste Biomass: A Case Study in the North of Colombia. Int. J. Chem. Tech. Res. 2018, 11, 33–40. [Google Scholar]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- van Kasteren, J.M.N.; Nisworo, A.P. A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour. Conserv. Recycl. 2007, 50, 442–458. [Google Scholar] [CrossRef] [Green Version]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.K.; Bai, F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2011.
- Wooley, R.J.; Putsche, V. Development of an ASPEN PLUS Physical Property Database for Biofuels Components; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1996.
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Zong, M.H.; Wu, H. Efficient lipid production with Trichosporonfermentans and its use for biodiesel preparation. Bioresour. Technol. 2008, 99, 7881–7885. [Google Scholar] [CrossRef]
- ASPEN PLUS Chemical Engineering Applications; Wiley: Hoboken, NJ, USA, 2017; ISBN 9788578110796.
- Bell, J.C.; Messerly, R.A.; Gee, R.; Harrison, A.; Rowley, R.L.; Wilding, W.V. Ternary liquid-liquid equilibrium of biodiesel compounds for systems consisting of a methyl ester + glycerin + water. J. Chem. Eng. Data 2013. [Google Scholar] [CrossRef]
- Luyben, W.L. Distillation Design and Control Using AspenTM Simulation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780471785255. [Google Scholar]
- Bertini, A.; Gelosia, M.; Cavalaglio, G.; Barbanera, M.; Giannoni, T.; Tasselli, G.; Nicolini, A.; Cotana, F. Production of Carbohydrates from Cardoon Pre-Treated by Acid-Catalyzed Steam Explosion and Enzymatic Hydrolysis. Energies 2019, 12, 4288. [Google Scholar] [CrossRef] [Green Version]
- Barbanera, M.; Castellini, M.; Tasselli, G.; Turchetti, B.; Cotana, F.; Buzzini, P. Prediction of the environmental impacts of yeast biodiesel production from cardoon stalks at industrial scale. Fuel 2021, 283, 118967. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Schmidt, J.E. Process simulation and economic assessment of hydrothermal pretreatment and enzymatic hydrolysis of multi-feedstock lignocellulose—Separate vs combined processing. Bioresour. Technol. 2018, 249, 835–843. [Google Scholar] [CrossRef]
- Damartzis, T.; Michailos, S.; Zabaniotou, A. Energetic assessment of a combined heat and power integrated biomass gasification–internal combustion engine system by using Aspen Plus®. Fuel Process. Technol. 2012, 95, 37–44. [Google Scholar] [CrossRef]
- Evans, C.T.; Ratledge, C. A comparison of the oleaginous yeast, Candida curvata, grown on different carbon sources in continuous and batch culture. Lipids 1983, 18, 623–629. [Google Scholar] [CrossRef]
- Babel, W.; Muller, R. Correlation between cell composition and carbon conversion efficiency in microbial growth: A theoretical study. Appl. Microbiol. Biotechnol. 1985, 22. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Peralta-Ruiz, Y.; González-Delgado, A.-D.; Kafarov, V. Evaluation of alternatives for microalgae oil extraction based on exergy analysis. Appl. Energy 2013, 101, 226–236. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Drogui, P. Cost, energy and GHG emission assessment for microbial biodiesel production through valorization of municipal sludge and crude glycerol. Bioresour. Technol. 2020, 297, 122404. [Google Scholar] [CrossRef]
- Xie, W.; Huang, X.; Li, H. Soybean oil methyl esters preparation using NaX zeolites loaded with KOH as a heterogeneous catalyst. Bioresour. Technol. 2007, 98, 936–939. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Mittelbach, M.; López, F.J. Kinetic Parameters Affecting the Alkali-Catalyzed Transesterification Process of Used Olive Oil. Energy Fuels 2004, 18, 1457–1462. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Cadoche, L.; López, G.D. Assessment of size reduction as a preliminary step in the production of ethanol from lignocellulosic wastes. Biol. Wastes 1989, 30, 153–157. [Google Scholar] [CrossRef]
- Szepessy, S.; Thorwid, P. Low Energy Consumption of High-Speed Centrifuges. Chem. Eng. Technol. 2018, 41, 2375–2384. [Google Scholar] [CrossRef] [Green Version]
- Milledge, J.J.; Heaven, S. Disc Stack Centrifugation Separation and Cell Disruption of Microalgae: A Technical Note. Environ. Nat. Resour. Res. 2011, 1. [Google Scholar] [CrossRef] [Green Version]
- Martis, R.; Al-Othman, A.; Tawalbeh, M.; Alkasrawi, M. Energy and Economic Analysis of Date Palm Biomass Feedstock for Biofuel Production in UAE: Pyrolysis, Gasification and Fermentation. Energies 2020, 13, 5877. [Google Scholar] [CrossRef]
- Sotoft, L.F.; Rong, B.-G.; Christensen, K.V.; Norddahl, B. Process simulation and economical evaluation of enzymatic biodiesel production plant. Bioresour. Technol. 2010, 101, 5266–5274. [Google Scholar] [CrossRef]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2003; ISBN 9789896540821. [Google Scholar]
- Garrett, D.E. Chemical Engineering Economics; Springer: Dordrecht, The Netherlands, 1989; ISBN 978-94-011-6546-4. [Google Scholar]
- Vinet, L.; Zhedanov, A. A ‘missing’ family of classical orthogonal polynomials. J. Phys. A Math. Theor. 2011, 44, 085201. [Google Scholar] [CrossRef]
- Parsons, S.; Abeln, F.; McManus, M.C.; Chuck, C.J. Techno-economic analysis (TEA) of microbial oil production from waste resources as part of a biorefinery concept: Assessment at multiple scales under uncertainty. J. Chem. Technol. Biotechnol. 2019, 94, 701–711. [Google Scholar] [CrossRef]
- Richardson, J.W.; Johnson, M.D.; Outlaw, J.L. Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. 2012, 1, 93–100. [Google Scholar] [CrossRef]
- Ratledge, C.; Cohen, Z. Microbial and algal oils: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Alabi, A.; Tampier, M.; Bibeau, E. Microalgae Technologies & Processes for Biofuels-Bioenergy Production in British Columbia: Current Technology, Suitability & Barriers to Implementation; Final Report Submitted to British Columbia Innovation Council; British Columbia Innovation Council: Vancouver, BC, Canada, 2009. [Google Scholar]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The Biochemistry and Molecular Biology of Lipid Accumulation in Oleaginous Microorganisms. Adv. Appl. Microb. 2002, 1–52. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Remer, D.S.; Chai, L.H. Design cost factors for scaling-up engineering equipment. Chem. Eng. Prog. 1990, 86, 77–82. [Google Scholar]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A.; Estevez, R. Acetone Prospect as an Additive to Allow the Use of Castor and Sunflower Oils as Drop-In Biofuels in Diesel/Acetone/Vegetable Oil Triple Blends for Application in Diesel Engines. Molecules 2020, 25, 2935. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A.; Luna, D.; Estévez, R. Biofuels from Diethyl Carbonate and Vegetable Oils for Use in Triple Blends with Diesel Fuel: Effect on Performance and Smoke Emissions of a Diesel Engine. Energies 2020, 13, 6584. [Google Scholar] [CrossRef]








| Component | Composition (Dry wt %) |
|---|---|
| Cellulose | 30.52 |
| Xylan | 14.52 |
| Mannan | 0.45 |
| Galactan | 1.06 |
| Arabinan | 1.15 |
| Acetate | 5.02 |
| Pectine | 4.67 |
| Lignin | 14.21 |
| Ash | 8.80 |
| Protein | 3.10 |
| Extractives | 16.51 |
| Moisture (bulk wt %) | 15.00 |
| Stoichiometry | Fractional Conversion of Component | Fractional Conversion |
|---|---|---|
| Cellulose + Water → Glucose | Cellulose | 0.041 |
| Cellulose → HMF + 2 Water | Cellulose | 0.006 |
| Xylan + Water → Xylose | Xylan | 0.386 |
| Xylan → Furfural + 2 Water | Xylan | 0.075 |
| Mannan + Water → Mannose | Mannan | 0.386 |
| Mannan → HMF + 2 Water | Mannan | 0.010 |
| Galactan + Water → Galactose | Galactan | 0.386 |
| Galactan → HMF + 2 Water | Galactan | 0.010 |
| Arabinan + Water → Arabinose | Arabinan | 0.386 |
| Arabinan → Furfural + 2 Water | Arabinan | 0.075 |
| Acetate → Acetic Acid | Acetate | 1.000 |
| Lignin → Soluble Lignin | Lignin | 0.050 |
| Stoichiometry | Fractional Conversion of Component | Fractional Conversion |
|---|---|---|
| Acetic Acid + Ammonia → Ammonium-Acetate | Acetic Acid | 0.037 |
| Sulfuric Acid + 2 Ammonia → Ammonium-Sulfate | Sulfuric Acid | 0.041 |
| Arabinose → TAR | Arabinose | 0.006 |
| Galactose → 1.2 TAR | Galactose | 0.356 |
| Mannose → 1.2 TAR | Mannose | 0.386 |
| Sulfuric Acid + 2 Ammonia → Ammonium-Sulfate | Ammonia | 1.000 |
| Stoichiometry | Fractional Conversion of Component | Fractional Conversion |
|---|---|---|
| Cellulose + Water → Glucose | Cellulose | 0.739 |
| Xylan + Water → Xylose | Xylan | 0.748 |
| Mannan + Water → Mannose | Mannan | 0.748 |
| Galactan + Water → Galactose | Galactan | 0.748 |
| Arabinan + Water → Arabinose | Arabinan | 0.748 |
| Stoichiometry | Fractional Conversion of Component | Fractional Conversion |
|---|---|---|
| Glucose + 3.41 Oxygen + 0.52 Aminoacids → 1.12 Microbial Biomass + 3.75 Carb. Dioxide + 4.41 Water + 0.01 Triolein | Glucose | 0.230 |
| Xylose + 2.41 Oxygen + 0.52 Aminoacids → 1.12 Microbial Biomass + 2.75 Carb. Dioxide + 3.41 Water + 0.01 Triolein | Xylose | 0.190 |
| Glucose + 1.2 Oxygen → 0.06 Triolein + 2.58 Carb. Dioxide + 2.88 Water | Glucose | 0.510 |
| Xylose + 0.2 Oxygen → 0.06 Triolein + 1.58 Carb. Dioxide + 1.88 Water | Xylose | 0.420 |
| Stoichiometry | Fractional Conversion of Component | Fractional Conversion |
|---|---|---|
| Triolein + 3 Chloroform → 3 M. Oleate + Glycerol | Triolein | 0.981 |
| 3 Sod. Hydroxide + Phosp. Acid → Tris. Phospate + 3 Water | Sod. Hydroxide | 1.000 |
| Aspen Plus Block | Heat Duty (MWh/y) |
|---|---|
| R1 | 2610 |
| R2 | 2202 |
| R4 | 838 |
| R9 | 13 |
| COL-1 (Reboiler) | 1826 |
| COL-2 (Reboiler) | 80 |
| COL-3 (Reboiler) | 115 |
| COL-4 (Reboiler) | 210 |
| Total | 7894 |
| Aspen Plus Block | Utility ID | Utility Duty (MWh/y) |
|---|---|---|
| R3 | Cooling water | 318.4 |
| R8 | Cooling water | 6.4 |
| COL-1 (Condenser) | Cooling water | 1515 |
| COL-2 (Condenser) | Cooling water | 59.2 |
| COL-3 (Condenser) | Cooling water | 108 |
| COL-4 (Condenser) | Cooling water | 22.9 |
| CEX3 | Cooling water | 183.2 |
| CEX4 | Cooling water | 2.4 |
| CEX5 | Cooling water | 13.6 |
| R6 | Refrigerant 1 | 4691 |
| R7 | Refrigerant 1 | 108 |
| CEX1 | Refrigerant 1 | 406 |
| CEX2 | Refrigerant 1 | 85.9 |
| Equipment purchased cost | 4,836,000 |
| Equipment installed cost | 7,737,600 |
| Total direct costs (TDC) | 7,737,600 |
| Prorateable expenses (10% of TDC) | 773,760 |
| Field expenses (10% of TDC) | 773,760 |
| Home office and construction fee (20% of TDC) | 1,547,520 |
| Project contingency (10% of TDC) | 773,760 |
| Other costs (10% of TDC) | 773,760 |
| Total indirect costs (TIC) | 4,642,560 |
| Fixed capital investment (FCI) | 12,380,160 |
| Working capital (5% of FCI) | 619,008 |
| Total capital investment (TCI) | 12,999,168 |
| Utilities | Utility Cost | Cost Unit | Rate | Rate Unit | Cost Per Hour (USD/h) |
|---|---|---|---|---|---|
| Cooling water | 2.12 × 10−7 | USD/kJ | 813,395 | kJ/h | 0.17 |
| Refrigerant 1 | 2.71 × 10−6 | USD/kJ | 209,811 | kJ/h | 0.57 |
| Raw Material | Amount (t/y) | Unit Cost (USD/t) | Total Cost (kUSD/y) |
|---|---|---|---|
| Biomass d | 10,000 | 40 | 400 |
| Sulfuric acid b | 233.7 | 67 | 15.7 |
| Ammonia b | 136 | 50 | 6.8 |
| Enzyme d | 6.6 | 3000 | 19.8 |
| Amino acids c | 250.4 | 800 | 200.3 |
| Methanol b | 80.9 | 211 | 17.1 |
| Chloroform b | 8.3 | 211 | 1.8 |
| Sodium hydroxide b | 4.4 | 495 | 2.1 |
| Potassium hydroxide b | 9.76 | 890 | 8.7 |
| Phosphoric acid b | 3.5 | 804 | 2.8 |
| Process water b | 55,136 | 0.20 | 11.1 |
| Air | 7272 | - | - |
| By-product | |||
| Glycerol a | 41.6 | 1500 | 62.4 |
| Trisodium phosphate a | 5.9 | 500 | 2.9 |
| Waste treatment | |||
| Liquid c | 62,488 | 0.10 | 6.2 |
| Utilities | 266,720 |
| Raw materials | 386,200 |
| Labor and supervision | 800,000 |
| Maintenance | 210,384 |
| Operating charges | 200,000 |
| Plant overhead | 50,519 |
| General and Administration | 100,872 |
| Total production cost (TPC) | 2,014,695 |
| Depreciation (10% of TCI) | 1,299,917 |
| Taxes (15% of TCI) | 1,949,875 |
| Total fixed costs (FC) | 3,249,792 |
| Byproduct sales (BS) | 91,600 |
| Manufacturing costs (MC) | 5,199,187 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellini, M.; Ubertini, S.; Barletta, D.; Baffo, I.; Buzzini, P.; Barbanera, M. Techno-Economic Analysis of Biodiesel Production from Microbial Oil Using Cardoon Stalks as Carbon Source. Energies 2021, 14, 1473. https://0-doi-org.brum.beds.ac.uk/10.3390/en14051473
Castellini M, Ubertini S, Barletta D, Baffo I, Buzzini P, Barbanera M. Techno-Economic Analysis of Biodiesel Production from Microbial Oil Using Cardoon Stalks as Carbon Source. Energies. 2021; 14(5):1473. https://0-doi-org.brum.beds.ac.uk/10.3390/en14051473
Chicago/Turabian StyleCastellini, Marco, Stefano Ubertini, Diego Barletta, Ilaria Baffo, Pietro Buzzini, and Marco Barbanera. 2021. "Techno-Economic Analysis of Biodiesel Production from Microbial Oil Using Cardoon Stalks as Carbon Source" Energies 14, no. 5: 1473. https://0-doi-org.brum.beds.ac.uk/10.3390/en14051473