Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes
Abstract
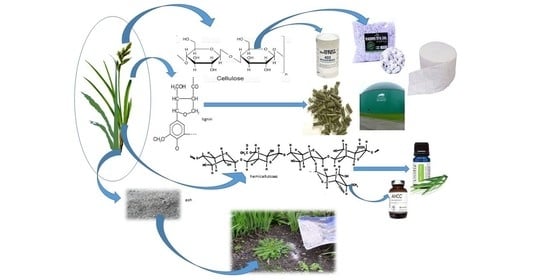
:1. Introduction
2. Materials and Methods
- Extractive contents were determined using 96% ethanol according to Soxhlet (TAPPI–T 204 cm-07) [24];
- Cellulose content was determined by the Seifert method with a mixture of acetylacetone and dioxane at an acid pH [25];
- Lignin content was determined by the Tappi method using 72% sulfuric acid (TAPPI–T 222 om-06) [26];
- Holocellulose content was assayed using sodium chlorite (TAPPI–T 9 wd-75) [27];
- The theoretical content of hemicellulose was calculated mathematically as a difference between holocellulose and cellulose contents;
- Contents of ash were determined according to the DIN 51731 standards [28].
- C is the heat capacity of the calorimeter, 12,783.69 (J/°C);
- Dt is the temperature rise in the main period (°C);
- k is a correction for heat exchange with the surroundings (°C);
- c is the sum of corrections for additional thermal effects (J);
- m is the mass of the fuel sample (g).
- is the average gross calorific value of solid fuel in the analytical state (Jg);
- The heat of vaporization of water at 25 °C is 24.42, corresponding to 1% of water in the fuel (J/g);
- Wa is the moisture content in the analytical sample of fuel (%);
- The analytical factor for the conversion of hydrogen content to water content is 8.94;
- Ha is the hydrogen content in the analytical sample of fuel, according to the PN-EN ISO 16948: 2015-07 [29].
3. Results
4. Discussion
5. Conclusions
- According to the hypothesis, Polish grasses from ecological sites are suitable for bioenergy conversion.
- The contents of cellulose, lignin and holocellulose do not differ from those in other annual plants. High contents of cellulose in reed canary grass and common bent indicate the potential for conversion of these species to produce cellulose as a valuable raw material.
- The above-average content of polysaccharides with a low degree of polymerization indicates the potential use of such plant biomass in biogas production. In this respect, three species seem to be of particular interest: Bromus inermis, Calamagrostis epigejos and Anthoxanthum odoratum.
- The studied grass species had high contents of extractives, which suggests that they may be sources of valuable active substances for use as dietary supplements or in cosmetics. The analyses show that the best species in this respect would be Agropyron repens, Bromus inermis and Arrhenatherum elatius.
- No correlation was found between contents of individual chemical components and the heat of combustion of the investigated grasses (data not shown). Neither the content of cellulose, nor that of lignin showed a definite effect on the heating value or heat of combustion. The best suited for use in energy generation are the species whose heating value exceeded 17.000 MJ/kg. These were Phalaris arundinacea, Calamagrostis epigejos, Phragmites australis, Agropyron repens and Bromus inermis.
- The higher contents of ash characteristics for grasses do not reduce the energy properties of these plants, while ash may additionally be used in soil fertilization. Among the investigated grass species, the most favorable in this respect were Agropyron repens, Bromus inermis and Arrhenatherum elatius.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EEA. National Monitoring, Reporting and Evaluation of Climate Change Adaptation in Europe; EEA Technical Report No 20/2015; European Environment Agency: København, Denmark, 2015; Available online: https://www.eea.europa.eu/publications/national-monitoring-reporting-and-evaluation (accessed on 1 November 2020).
- Czekała, W.; Bartnikowska, S.; Dach, J.; Janczak, D.; Smurzyńska, A.; Kozłowski, K.; Bugała, A.; Lewicki, A.; Cieślik, M.; Typańska, D.; et al. The energy value and economic efficiency of solid biofuels produced from digestate and sawdust. Energy 2018, 159, 1118–1122. [Google Scholar] [CrossRef]
- Cieślik, M.; Dach, J.; Lewicki, A.; Smurzyńska, A.; Janczak, D.; Pawlicka-Kaczorowska, J.; Boniecki, P.; Cyplik, P.; Czekała, W.; Jóźwiakowski, K. Methane fermentation of the maize straw silage under meso- and thermophilic conditions. Energy 2016, 115, 1495–1502. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize Straw as a Valuable Energetic Material for Biogas Plant Feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk-Juśko, A.; Kościk, B.; Jóźwiakowski, K.; Marczuk, A.; Zarajczyk, J.; Kowalczuk, J.; Szmigielski, M.; Sagan, A. Effects of biochemical and thermochemical conversion of sorghum biomass to usable energy/Efekty biochemicznej i termochemicznej konwersji biomasy sorga (Sorghum bicolor Moench.) na energię użytkową. Przem. Chem. 2015, 1, 212–214. [Google Scholar] [CrossRef]
- Pochwatka, P.; Kowalczyk-Jusko, A.; Mazur, A.; Janczak, D.; Pulka, J.; Dach, J.; Mazurkiewicz, J. Energetic and Economic Aspects of Biogas Plants Feed with Agriculture Biomass. In Proceedings of the 2020 4th International Conference on Green Energy and Applications, ICGEA 2020, Singapore, 7–9 March 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 130–133. [Google Scholar]
- Wannasek, L.; Ortner, M.; Amon, B.; Amon, T. Sorghum, a sustainable feedstock for biogas production? Impact of climate, variety and harvesting time on maturity and biomass yield. Biomass Bioenergy 2017, 106, 137–145. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Pochwatka, P.; Zaborowicz, M.; Czekała, W.; Mazurkiewicz, J.; Mazur, A.; Janczak, D.; Marczuk, A.; Dach, J. Energy value estimation of silages for substrate in biogas plants using an artificial neural network. Energy 2020, 202. [Google Scholar] [CrossRef]
- Perzon, M. Emissions of organic compounds from the combustion of oats—A comparison with softwood pellets. Biomass Bioenergy 2010, 34, 828–837. [Google Scholar] [CrossRef]
- Czekała, W.; Dach, J.; Janczak, D.; Smurzyńska, A.; Kwiatkowska, A.; Kozłowski, K. Influence of maize straw content with sewage sludge on composting process. J. Water Land Dev. 2016, 30, 43–49. [Google Scholar] [CrossRef]
- Janczak, D.; Malińska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Waszkielis, K.M.; Wronowski, R.; Chlebus, W.; Białobrzewski, I.; Dach, J.; Pilarski, K.; Janczak, D. The effect of temperature, composition and phase of the composting process on the thermal conductivity of the substrate. Ecol. Eng. 2013, 61, 354–357. [Google Scholar] [CrossRef]
- Białobrzewski, I.; Mikš-Krajnik, M.; Dach, J.; Markowski, M.; Czekała, W.; Głuchowska, K. Model of the sewage sludge-straw composting process integrating different heat generation capacities of mesophilic and thermophilic microorganisms. Waste Manag. 2015, 43, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Pochwatka, P.; Kowalczyk-Juśko, A.; Sołowiej, P.; Wawrzyniak, A.; Dach, J. Biogas Plant Exploitation in a Middle-Sized Dairy Farm in Poland: Energetic and Economic Aspects. Energies 2020, 13, 6058. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- GUS. Rocznik Statystyczny Rolnictwa; Główny Urząd Statystyczny: Warszawa, Poland, 2016. (In Polish) [Google Scholar]
- Dubert, F.; Górski, F. Cultivation of “Energy Plants”—Benefits and Dangers; Annual Report 2010; Polish Academy of Sciences: Warszawa, Poland, 2010; pp. 69–73. [Google Scholar]
- Cintas, O.; Berndes, G.; Cowie, A.L.; Egnell, G.; Holmström, H.; Marland, G.; Ågren, G.I. Carbon balances of bioenergy systems using biomass from forests managed with long rotations: Bridging the gap between stand and landscape assessments. GCB Bioenergy 2017, 9, 1238–1251. [Google Scholar] [CrossRef] [Green Version]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M. Energy Value of Yield and Biomass Quality of Poplar Grown in Two Consecutive 4-Year Harvest Rotations in the North-East of Poland. Energies 2020, 13, 1495. [Google Scholar] [CrossRef] [Green Version]
- Wicke, B.; Kluts, I.; Lesschen, J.P. Bioenergy Potential and Greenhouse Gas Emissions from Intensifying European Temporary Grasslands. Land 2020, 9, 457. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between Biogas Yield and Chemical Composition of Grassland Plant Species. Energy Fuels 2015, 29, 7221–7229. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Ind. Crop. Prod. 2017, 109, 227–232. [Google Scholar] [CrossRef]
- TAPPI. T 204 cm-07—Solvent Extractives of Wood and Pulp; Standards Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2007. [Google Scholar]
- Seifert, K. Zur Frage der Cellulose-Schnellbestimmung nach der Acetylaceton-Methode. Das Pap. 1960, 14, 104–106. (In German) [Google Scholar]
- TAPPI. T-222 om-06—Lignin in Wood and Pulp; Standards Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2006. [Google Scholar]
- TAPPI. T 9 wd-75—Holocellulose in Wood; Standards Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 1975. [Google Scholar]
- DIN. DIN 51731. Testing of Solid Fuels—Compressed Untreated Wood—Requirements and Testing; Deutsches Institut für Normung E.V. (German National Standard): Berlin, Germany, 1996. [Google Scholar]
- ISO. PN-EN ISO 16948: 2015-07 Solid Biofuels. Total Carbon, Hydrogen and Nitrogen Determination; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Kupryaniuk, K.; Oniszczuk, T.; Combrzyński, M.; Dach, J.; Czekała, W. Process Efficiency and Energy Consumption during the Extrusion of Lignocellulosic Materials. Proc. IOP Conf. Ser. Earth Environ. Sci. 2020, 505, 012040. [Google Scholar] [CrossRef]
- Gizińska-Górna, M.; Czekała, W.; Jóźwiakowski, K.; Lewicki, A.; Dach, J.; Marzec, M.; Pytka, A.; Janczak, D.; Kowalczyk-Juśko, A.; Listosz, A. The possibility of using plants from hybrid constructed wetland wastewater treatment plant for energy purposes. Ecol. Eng. 2016, 95, 534–541. [Google Scholar] [CrossRef]
- Prosiński, S. Chemia Drewna (Wood Chemistry); PWRiL: Warszawa, Poland, 1984. (In Polish) [Google Scholar]
- Rowell, R.M.; Han, J.S.; Bisen, S.S. Changes in fiber properties during the growing season. In Paper and Composite from Agro-Based Resources; Rowell, R.M., Young, R.A., Rowell, J.K., Eds.; Lewis Publishers: Boca Raton, FL, USA; New York, NY, USA; London, UK; Tokyo, Japan, 1997; pp. 23–37. [Google Scholar]
- Baeza, J.; Freer, J. Chemical Characterization of Wood and Its Components. In Wood and Cellulosic Chemistry, 2nd ed.; Hon, N.-S., Shiraishi, N., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; p. 275. [Google Scholar]
- Waliszewska, B.; Prądzyński, W. Basic chemical analysis and polymerization level of cellulose in the year-old and the multiyear shrubby willows growing by the A-2 motorway. Proceedings of National Symposium, Biological Reactions of Trees to Industrial Pollution, Kórnik, Poland, 29 May–1 June 2001; pp. 725–732. [Google Scholar]
- Doczekalska, B.; Bartkowiak, M.; Waliszewska, B.; Orszulak, G.; Cerazy-Waliszewska, J.; Pniewski, T. Characterization of Chemically Activated Carbons Prepared from Miscanthus and Switchgrass Biomass. Materials 2020, 13, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waliszewska, B.; Janyszek, M.; Grzelak, M.; Gaweł, E. Characteristic of thermochemical parameters of aboveground parts of selected sedges (Carex L Cyperaceae). IOSR J. Agric. Vet. Sci. 2013, 5, 1–6. [Google Scholar] [CrossRef]
- Ansah, T.; Osafo, E.; Hansen, H. Herbage yield and chemical composition of four varieties of Napier (Pennisetum purpureum) grass harvested at three different days after planting. Agric. Biol. J. N. Am. 2010, 1, 923–929. [Google Scholar] [CrossRef]
- Murawski, M.; Grzelak, M.; Waliszewska, B.; Knioła, A.; Czekała, W. Energy value and yielding from extensively used meadows. Fragm. Agron. 2015, 32, 71–78. [Google Scholar]
- Krzyżaniak, M.; Stolarski, M.J.; Waliszewska, B.; Szczukowski, S.; Tworkowski, J.; Załuski, D.; Śnieg, M. Willow biomass as feedstock for an integrated multi-product biorefinery. Ind. Crop. Prod. 2014, 58, 230–237. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Tworkowski, J.; Wróblewska, H.; Krzyżaniak, M. Short rotation willow coppice biomass as an industrial and energy feedstock. Ind. Crop. Prod. 2011, 33, 217–223. [Google Scholar] [CrossRef]
- Chen, Q.; Endo, T.; Wang, Q. Characterization of bamboo after ionic liquid-H2O pretreatment for the pyrolysis process. Bioresources 2015, 10, 2797–2808. [Google Scholar] [CrossRef] [Green Version]
- Bedoić, R.; Čuček, L.; Ćosić, B.; Krajnc, D.; Smoljanić, G.; Kravanja, Z.; Ljubas, D.; Pukšec, T.; Duić, N. Green biomass to biogas—A study on anaerobic digestion of residue grass. J. Clean. Prod. 2019, 213, 700–709. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Vayenas, D.; Lyberatos, G. Biogas Production from Physicochemically Pretreated Grass Lawn Waste: Comparison of Different Process Schemes. Molecules 2020, 25, 296. [Google Scholar] [CrossRef] [Green Version]
- Piekarczyk, M.; Kotwica, K.; Jaskulski, D. The elemental composition of ash from straw and hay in the context of their agricultural utilization. Acta Sci. Pol. Agric. 2011, 10, 97–104. [Google Scholar]
- Kalembasa, D. The amount and chemical composition of ash obtained from biomass of energy crops. Acta Agrophys. 2006, 7, 909–914. [Google Scholar]
- Grzelak, M.; Gaweł, E.; Waliszewska, B.; Janyszek, M.; Wrońska-Pilarek, D.; Murawski, M.; Knioła, A. Floristic and habitat variability, nature and energy value of selected sedge communities. Steciana 2017, 20, 233–238. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A. Popiół z różnych roślin energetycznych (Ash from different energy crops). Proc. ECOpole 2009, 3, 159–164. (In Polish) [Google Scholar]
- Frączek, J.; Mudryk, K.; Wróbel, M. Rożnik przerośnięty Silphium perfoliatum L.—Źródło biomasy do produkcji biopaliw stałych (Cup plant Silphium perfoliatum L.—A source of biomass for the production of solid biofuels). Inż. Rol. 2011, 6, 21–27. (In Polish) [Google Scholar]
- Komorowicz, M.; Wróblewska, H.; Pawłowski, J. Skład chemiczny i właściwości energetyczne biomasy z wybranych surowców odnawialnych (Chemical composition and heating value of biomass from selected renewable raw materials). Ochr. Środ. Zasobów Nat. 2009, 40, 402–410. (In Polish) [Google Scholar]
- Kowalczyk-Juśko, A. The influence of the ash from the biomass on the power boiler pollution. J. Ecol. Eng. 2017, 18, 200–204. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Kościk, B. Produkcja biomasy miskanta cukrowego i spartiny preriowej w zróżnicowanych warunkach glebowych oraz możliwości jej konwersji na energię (Production of biomass from Amur silvergrass and prairie cordgrass under various soil conditions and potential conversion of such biomass to energy). Biul. IHAR 2004, 234, 213–218. [Google Scholar]
- Martyniak, D. Perz wydłużony—Nowa roślina na cele energetyczne (Tall wheatgrass—A new energy crop). Agro Serwis 2009, 18, 76–77. [Google Scholar]
- Stolarski, M.; Mleczek, M.; Szczukowski, S.; Goliński, P.; Waliszewska, B.; Szentner, K.; Rutkowski, P.; Krzyżaniak, M. Characteristics of Thermophysical Parameters of Selected Salix Taxa with Elemental Analysis. Int. J. Green Energy 2015, 12, 1272–1279. [Google Scholar] [CrossRef]
- Mirowski, T.; Mokrzycki, E.; Uliasz-Bocheńczyk, A. Energetyczne Wykorzystanie Biomasy (Utilisation of Biomass for Energy Generation); Wydawnictwo IGSMiE PAN: Kraków, Poland, 2018. (In Polish) [Google Scholar]
- Kołodziej, B.; Matyka, M. Odnawialne Źródła Energii. Rolnicze Surowce Energetyczne (Renewable Energy Sources. Agricultural Energy Resources); PWRiL: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Kowalczyk-Juśko, A. Biometric and energetic parameters of cordgrass (Spartina pectinata L.) in the first three years of growth. Probl. Agric. Eng. 2010, 2, 69–77. [Google Scholar]

| Grass Species | Contents [%] DM | ||||
|---|---|---|---|---|---|
| Extractives | Cellulose | Lignin | Holocellulose | Hemicellulose | |
| Phalaris arundinacea L. | 12.77 ± 0.22 c | 38.68 ± 0.01 d | 15.42 ± 0.01 a | 70.39 ± 0.89 b | 31.71 ± 1.46 abcd |
| Phragmites australis (Cav.) Trin. ex Steud | 14.46 ± 0.06 d | 35.05 ± 0.14 b | 21.99 ± 0.15 f | 65.32 ± 0.23 a | 30.27 ± 0.15 a |
| Dacylis glomerata L. | 10.59 ± 0.15 ab | 37.71 ± 0.41 cd | 19.33 ± 0.05 d | 69.19 ± 1.29 b | 31.48 ± 1.13 abc |
| Arrhenatherum elatius | 15.00 ± 0.17 d | 35.46 ± 0.20 b | 17.54 ± 0.15 c | 68.63 ± 0.09 b | 33.17 ± 0.25 cde |
| Bromus inermis Leyss. | 18.26 ± 0.05 e | 35.6 ± 0.51 b | 16.5 ± 0.60 b | 69.31 ± 0.95 b | 33.71 ± 1.15 e |
| Agrostis capillaris L. | 11.29 ± 0.44 b | 38.29 ± 0.18 d | 20.48 ± 0.14 e | 69.37 ± 0.39 b | 31.08 ± 0.39 ab |
| Calamagrostis epigejos L. (Roth) | 9.42 ± 0.01 a | 35.35 ± 0.19 b | 20.96 ± 0.09 e | 69.01 ± 0.43 b | 33.66 ± 0.9 de |
| Agropyron repens L. | 15.52 ± 1.07 d | 33.38 ± 1.35 a | 18.76 ± 0.19 d | 64.22 ± 0.52 a | 30.84 ± 0.84 ab |
| Anthoxanthum odoratum L. | 9.72 ± 0.28 a | 35.18 ± 0.28 b | 17.68 ± 0.04 c | 69.49 ± 0.48 b | 34.31 ± 0.20 e |
| Holcus lanatus L. | 13.07 ± 0.17 c | 36.,43 ± 0.40 c | 17.18 ± 0.16 c | 69.00 ± 0.55 b | 32.57 ± 0.32 bcde |
| Grass Species | Moisture Content (%) | Heat of Combustion (MJ/kg) | Heating Value (MJ/kg) |
|---|---|---|---|
| Phalaris arundinacea L. | 6.4 ± 0.02 | 18.757 ± 0.031 | 17.293 ± 0.030 |
| Phragmites australis (Cav.) Trin. ex Steud | 6.7 ± 0.09 | 18.842 ± 0.032 | 17.386 ± 0.031 |
| Dacylis glomerata L. | 6.2 ± 0.05 | 17.598 ± 0.019 | 16.129 ± 0.019 |
| Arrhenatherum elatius | 6.7 ± 0.05 | 18.455 ± 0.037 | 16.989 ± 0.036 |
| Bromus inermis Leyss. | 6.2 ± 0.03 | 18.707 ± 0.010 | 17.231 ± 0.010 |
| Agrostis capillaris L. | 6.4 ± 0.01 | 17.527 ± 0.001 | 16.066 ± 0.002 |
| Calamagrostis epigejos L. (Roth) | 6.4 ± 0.01 | 19.496 ± 0.032 | 18.037 ± 0.032 |
| Agropyron repens L. | 6.6 ± 0.05 | 19.252 ± 0.037 | 17.793 ± 0.036 |
| Anthoxanthum odoratum L. | 6.3 ± 0.06 | 17.912 ± 0.038 | 16.436 ± 0.036 |
| Holcus lanatus L. | 6.7 ± 0.05 | 17.502 ± 0.039 | 16.029 ± 0.038 |
| Grass Species | H (%) DM | Grass Species | H [%] DM |
|---|---|---|---|
| Phalaris arundinacea L. | 6.17 ± 0.04 | Agrostis capillaris L. | 6.04 ± 0.04 |
| Phragmites australis (Cav.) Trin. ex Steud | 5.83 ± 0.08 | Calamagrostis epigejos L. (Roth) | 6.08 ± 0.09 |
| Dacylis glomerata L. | 5.90 ± 0.08 | Agropyron repens L. | 6.07 ± 0.03 |
| Arrhenatherum elatius | 6.02 ± 0.06 | Anthoxanthum odoratum L. | 6.04 ± 0.06 |
| Bromus inermis Leyss. | 6.03 ± 0.02 | Holcus lanatus L. | 5.99 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. https://0-doi-org.brum.beds.ac.uk/10.3390/en14061669
Waliszewska B, Grzelak M, Gaweł E, Spek-Dźwigała A, Sieradzka A, Czekała W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies. 2021; 14(6):1669. https://0-doi-org.brum.beds.ac.uk/10.3390/en14061669
Chicago/Turabian StyleWaliszewska, Bogusława, Mieczysław Grzelak, Eliza Gaweł, Agnieszka Spek-Dźwigała, Agnieszka Sieradzka, and Wojciech Czekała. 2021. "Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes" Energies 14, no. 6: 1669. https://0-doi-org.brum.beds.ac.uk/10.3390/en14061669