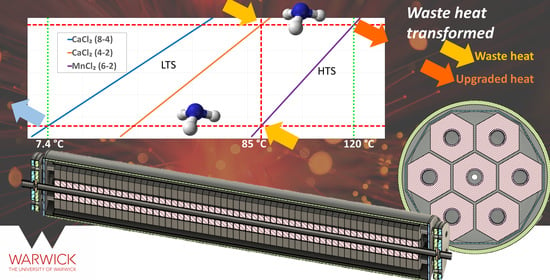
Resorption Thermal Transformer Generator Design
Abstract
:1. Introduction
1.1. Working Concept
- The first phase is the high-pressure phase. Waste heat is delivered to the low temperature salt, the salt desorbs refrigerant, and the pressure rises in the system. Ammonia begins to adsorb in the high-temperature bed and the temperatures in both beds climb the Clapeyron lines until at the Phigh condition in Figure 2. At this point, high-temperature (useful) heat is recovered and returned to the industrial process. The transition between pressures takes place over a short period, and the heat is delivered over an extensive period.
- The second phase resets the system. Cooling water is delivered to the low-temperature bed initiating ammonia adsorption, and the system pressure begins to fall. The beds drop down the Clapeyron lines to the low-pressure condition. Whilst this occurs, the waste heat is fed to the high-temperature bed to provide endothermic heat. The process continues at low pressure until the exothermic adsorption reaction is complete in the low-temperature salt reactor.
1.2. Ammonia-Salt Reactions
1.2.1. Introduction to the Thermodynamic Characteristics and Reaction Kinetics
1.2.2. Testing Methods from Literature
1.2.3. Modelling Reaction Kinetics
- –
- Reaction (I)
- –
- Reaction (II)
- –
- Reaction (I)
- –
- Reaction (II)
2. Materials and Methods
2.1. Clausius-Clapeyron Lines for Envelope of Operation
2.2. Large Temperature Jump and Experimental Results
2.3. Modelling Calcium Chloride Results and Obtaining Parameters for Reaction Kinetics
3. Analysis and Results
3.1. Salt Categorisation and Calcium Chloride Test Results
3.2. Model Results and Identified Constants for Reaction Kinetics
3.3. COP Calculation
3.4. Specific Mean Power and Peak Delivery
4. Discussion
4.1. Optimisation
4.2. Design Remarks
4.3. Challenges and Next Steps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Abbreviations
| Arrhenius factor (varies) | |
| COP | coefficient of performance |
| concentration of component A (mol m−3) | |
| kinetic coefficient (s−1) | |
| 𝐸,𝐸′ | pseudo-activation energy (K−1) |
| ENG | expanded natural graphite |
| HT, HTS | high temperature, high-temperature salt |
| ∆𝐻 | enthalpy term (J/kmol) |
| ITC | isosteric temperature change |
| 𝑘, 𝑘0 | kinetic coefficient (s−1) |
| LT, LTS | low temperature, low-temperature salt |
| LTJ | large temperature jump |
| 𝑛, 𝑛′ | pseudo-order of reaction, and second reaction |
| 𝑚, 𝑚′ | pseudo-order of reaction, and second reaction |
| 𝑚𝑆𝐴𝐿𝑇 | mass of salt (kg) |
| 𝑃, 𝑝 | pressure (Pa) |
| 𝑄 | heat (J) |
| 𝑅 | gas constant (J K−1 mol−1) |
| ∆𝑆 | entropy term (J kmol−1 K−1) |
| SMP | specific mean power (W m−3) |
| 𝑇 | temperature (K) |
| 𝑡 | time (s) |
| 𝑋 | conversion |
| 𝑌 | conversion of 2nd reaction |
Subscripts
| salt in form A | |
| 𝐴𝐵 | change from form A to B |
| 𝑎𝑑𝑠., 𝑎𝑑𝑠𝑜𝑟𝑝𝑡𝑖𝑜𝑛 | adsorption reaction term |
| 𝐵 | salt in form B |
| 𝐵𝐶 | change from form B to C |
| 𝐶 | salt in form C |
| 𝑑 | desorption |
| 𝑑𝑒𝑠., 𝑑𝑒𝑠𝑜𝑟𝑝𝑡𝑖𝑜𝑛 | desorption reaction term |
| 𝑒 | equilibrium conditions |
| ℎ, high | higher conditions (temperature or pressure) |
| ℎ𝑒𝑎𝑡 | reaction heat term |
| ℎ𝑒𝑎𝑡𝑖𝑛𝑔 | sensible heating term |
| 𝑙, low | lower conditions (temperature or pressure) |
| 𝑚 | middle conditions (temperature), waste heat temperature |
| 𝑅𝐸𝐴𝐶𝑇 | reaction term (heat of reaction) |
| 𝑠 | adsorption |
Appendix A


Appendix B


Appendix C

Appendix D

Appendix E

Appendix F

Appendix G

Appendix H

Appendix I

Appendix J


Appendix K
| Index | Tube OD (Cell ID mm) | Composite OD (mm) | COP | Volume of Composite (m3) |
|---|---|---|---|---|
| (a) | 25.4 (1”) | 36 | 0.197 | 0.00051 |
| (b) | 25.4 (1”) | 40 | 0.238 | 0.00075 |
| (c) | 19.05 (3/4”) | 36 | 0.293 | 0.00073 |
| (d) | 19.05 (3/4”) | 40 | 0.317 | 0.00097 |
| (e) | 12.7 (1/2”) | 33.6 * | 0.356 | 0.00076 |
References
- BEIS. UK Hydrogen Strategy. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1011283/UK-Hydrogen-Strategy_web.pdf (accessed on 5 March 2022).
- Hinmers, S.; Critoph, R.E. Modelling the Ammoniation of Barium Chloride for Chemical Heat Transformations. Energies 2019, 12, 4404. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, G.H.; Hinmers, S.; Critoph, R.E.; van der Pal, M. Ammonium Chloride (NH4Cl)—Ammonia (NH3): Sorption Characteristics for Heat Pump Applications. Energies 2021, 14, 6002. [Google Scholar] [CrossRef]
- Vasiliev, L.; Mishkinis, D.; Antukh, A.; Kulakov, A. Resorption heat pump. Appl. Therm. Eng. 2004, 24, 1893–1903. [Google Scholar] [CrossRef]
- Bao, H.; Wang, R.; Oliveira, R.; Li, T. Resorption system for cold storage and long-distance refrigeration. Appl. Energy 2012, 93, 479–487. [Google Scholar] [CrossRef]
- Goetz, V.; Elie, F.; Spinner, B. The structure and performance of single effect solid-gas chemical heat pumps. Heat Recover. Syst. CHP 1993, 13, 79–96. [Google Scholar] [CrossRef]
- Cudok, F.; Giannetti, N.; Ciganda, J.L.C.; Aoyama, J.; Babu, P.; Coronas, A.; Fujii, T.; Inoue, N.; Saito, K.; Yamaguchi, S.; et al. Absorption heat transformer-state-of-the-art of industrial applications. Renew. Sustain. Energy Rev. 2021, 141, 110757. [Google Scholar] [CrossRef]
- Metcalf, S.; Critoph, R.; Tamainot-Telto, Z. Optimal cycle selection in carbon-ammonia adsorption cycles. Int. J. Refrig. 2012, 35, 571–580. [Google Scholar] [CrossRef]
- Korhammer, K.; Neumann, K.; Opel, O.; Ruck, W.K. Micro-scale Thermodynamic and Kinetic Analysis of a Calcium Chloride Methanol System for Process Cooling. Energy Procedia 2017, 105, 4363–4369. [Google Scholar] [CrossRef]
- Offenhartz, P.O.; Rye, T.; Malsberber; Schwartz, D. Methanol-Based Heat Pump for Solar Heating, Cooling and Storage, Phase III; EIC Laboratories, Inc.: Newton, MA, USA, 1981. [Google Scholar]
- Aristov, Y. Optimal adsorbent for adsorptive heat transformers: Dynamic considerations. Int. J. Refrig. 2009, 32, 675–686. [Google Scholar] [CrossRef]
- Critoph, R. Activated carbon adsorption cycles for refrigeration and heat pumping. Carbon 1989, 27, 63–70. [Google Scholar] [CrossRef]
- van der Pal, M.; Critoph, R.E. Performance of CaCl2-reactor for application in ammonia-salt based thermal transformers. Appl. Therm. Eng. 2017, 126, 518–524. [Google Scholar] [CrossRef]
- Neveu, P.; Castaing, J. Solid-gas chemical heat pumps: Field of application and performance of the internal heat of reaction recovery process. Heat Recover. Syst. CHP 1993, 13, 233–251. [Google Scholar] [CrossRef]
- Moundanga-Iniamy, M.; Touzain, P. The Reaction between Ammonia and Magnesium Chloride Graphite Intercalation Compounds. Mater. Sci. Forum 1992, 91–93, 823–828. [Google Scholar] [CrossRef]
- Touzain, P.; Moundanga-Iniamy, M. Thermochemical Heat Transformation: Study of The Ammonia/Magnesium Chloride-Gic Pair in A Laboratory Pilot. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1994, 245, 243–248. [Google Scholar] [CrossRef]
- Touzain, P.; El Atifi, A.; Moundanga-Iniamy, M. Reaction of Metal Chloride Graphite Intercalation Compounds with Ammonia. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1994, 245, 231–236. [Google Scholar] [CrossRef]
- Mazet, N.; Amouroux, M.; Spinner, B. Analysis and Experimental Study of the Transformation of a Non-Isothermal Solid/Gas Reacting Medium. Chem. Eng. Commun. 1991, 99, 155–174. [Google Scholar] [CrossRef]
- Mazet, N.; Amouroux, M. Analysis of Heat Transfer in a Non-Isothermal Solid-Gas Reacting Medium. Chem. Eng. Commun. 1991, 99, 175–200. [Google Scholar] [CrossRef]
- Zhong, Y.; Critoph, R.; Thorpe, R.; Tamainot-Telto, Z. Dynamics of BaCl2–NH3 adsorption pair. Appl. Therm. Eng. 2009, 29, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- An, G.; Wang, L.; Zhang, Y. Overall evaluation of single- and multi-halide composites for multi-mode thermal-energy storage. Energy 2020, 212, 118756. [Google Scholar] [CrossRef]
- An, G.; Li, Y.; Wang, L.; Gao, J. Wide applicability of analogical models coupled with hysteresis effect for halide/ammonia working pairs. Chem. Eng. J. 2020, 394, 125020. [Google Scholar] [CrossRef]
- Zhong, Y.; Critoph, R.; Thorpe, R.; Tamainot-Telto, Z.; Aristov, Y. Isothermal sorption characteristics of the BaCl2–NH3 pair in a vermiculite host matrix. Appl. Therm. Eng. 2007, 27, 2455–2462. [Google Scholar] [CrossRef]
- Dawoud, B.; Aristov, Y. Experimental study on the kinetics of water vapor sorption on selective water sorbents, silica gel and alumina under typical operating conditions of sorption heat pumps. Int. J. Heat Mass Transf. 2003, 46, 273–281. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Tokarev, M.M. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation: 4. Dynamics of quasi-isobaric ammonia sorption and desorption on BaCl2/vermiculite. Appl. Therm. Eng. 2011, 31, 566–572. [Google Scholar] [CrossRef]
- Aristov, Y.I. Experimental and numerical study of adsorptive chiller dynamics: Loose grains configuration. Appl. Therm. Eng. 2013, 61, 841–847. [Google Scholar] [CrossRef]
- Metcalf, S.; Rivero-Pacho, A.; Critoph, R. Design and Large Temperature Jump Testing of a Modular Finned-Tube Carbon–Ammonia Adsorption Generator for Gas-Fired Heat Pumps. Energies 2021, 14, 3332. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, E.A.; Dutta, P.; Murthy, S.S.; Aristov, Y.; Tokrev, M.; Li, T.; Wang, R. Ammoniated salt based solid sorption thermal batteries: A comparative study. Appl. Therm. Eng. 2021, 191, 116875. [Google Scholar] [CrossRef]
- Hinmers, S.; Atkinson, G.; Critoph, R.; van der Pal, M. Modelling and Analysis of Ammonia Sorption Reactions in Halide Salts. Int. J. Refrig. 2022. [Google Scholar] [CrossRef]
- An, G.; Wang, L.; Gao, J.; Wang, R. A review on the solid sorption mechanism and kinetic models of metal halide-ammonia working pairs. Renew. Sustain. Energy Rev. 2018, 91, 783–792. [Google Scholar] [CrossRef]
- Veselovskaya, J.; Critoph, R.; Thorpe, R.; Metcalf, S.; Tokarev, M.; Aristov, Y. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation: 3. Testing of “BaCl2/vermiculite” composite in a lab-scale adsorption chiller. Appl. Therm. Eng. 2010, 30, 1188–1192. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Tokarev, M.M.; Grekova, A.D.; Gordeeva, L.G. Novel ammonia sorbents ‘‘porous matrix modified by active salt” for adsorptive heat transformation: 6. The ways of adsorption dynamics enhancement. Appl. Therm. Eng. 2012, 37 (Suppl. C), 87–94. [Google Scholar] [CrossRef]
- Lebrun, M.; Spinner, B. Models of heat and mass transfers in solid—gas reactors used as chemical heat pumps. Chem. Eng. Sci. 1990, 45, 1743–1753. [Google Scholar] [CrossRef]
- Lebrun, M.; Spinner, B. Simulation for the development of solid—gas chemical heat pump pilot plants Part I. simulation and dimensioning. Chem. Eng. Process. Process Intensif. 1990, 28, 55–66. [Google Scholar] [CrossRef]
- Lebrun, M. Simulation for the development of solid—gas chemical heat pump pilot plants Part II. simulation and optimization of Discontinuous and pseudo-continuous operating cycles. Chem. Eng. Process. Process Intensif. 1990, 28, 67–77. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Lu, H.-B.; Mazet, N.; Spinner, B. Modelling of gas-solid reaction—Coupling of heat and mass transfer with chemical reaction. Chem. Eng. Sci. 1996, 51, 3829–3845. [Google Scholar] [CrossRef]
- Lu, H.-B.; Mazet, N.; Coudevylle, O.; Mauran, S. Comparison of a general model with a simplified approach for the transformation of solid-gas media used in chemical heat transformers. Chem. Eng. Sci. 1997, 52, 311–327. [Google Scholar] [CrossRef]
- Lepinasse, E.; Goetz, V.; Crosat, G. Modelling and experimental investigation of a new type of thermochemical transformer based on the coupling of two solid-gas reactions. Chem. Eng. Process. Process Intensif. 1994, 33, 125–134. [Google Scholar] [CrossRef]
- Gluesenkamp, K.R.; Frazzica, A.; Velte, A.; Metcalf, S.; Yang, Z.; Rouhani, M.; Blackman, C.; Qu, M.; Laurenz, E.; Rivero-Pacho, A.; et al. Experimentally Measured Thermal Masses of Adsorption Heat Exchangers. Energies 2020, 13, 1150. [Google Scholar] [CrossRef] [Green Version]












| Salt | BaCl2 | CaCl2 | MnCl2 | |
|---|---|---|---|---|
| NH3 mole change | 8-0 | 8-4 | 4-2 | 6-2 |
| (J/kmol) | 48,924,790 | 36,365,790 | 41,202,320 | 58,196,253 |
| (J/kmol) | 37,360,200 | 32,844,620 | 31,699,720 | 36,611,107 |
| (J/kmol K) | 263,993 | 217,432 | 224,432 | 253,641 |
| (J/kmol K) | 229,454 | 208,302 | 202,390 | 202,865 |
| (J/kmol) | 40,745,437 | 42,080,324 | 39,948,772 | 42,523,900 |
| Reference | [29] | (This paper) | [29] | |
| Salt | MnCl2 | BaCl2 | CaCl2 | CaCl2 |
|---|---|---|---|---|
| NH3 mole change | 6–2 | 8–0 | 8–4 | 4–2 |
| adsorption | 2 | 2 | 2 | 2 |
| adsorption | 3 | 0.1 | 1.5 | 3 |
| desorption | 3 | 2 | 2 | 1.5 |
| desorption | 3 | 3 | 3 | 2.5 |
| Active fraction | 0.8 | 0.78 | 0.8 | 0.8 |
| Source | [29] | [29] | (This paper) | (This paper) |
| Sorption Pair (mols NH3 Reacting) | Corresponding Diagram Figure 9 | COP |
|---|---|---|
| Calcium chloride (8-4) + Magnesium chloride (6-2) | (a) | 0.20 |
| Barium chloride (8-0) + Calcium chloride (4-2) | (b) | 0.34 |
| Calcium chloride (8-4-2) + Magnesium chloride (6-2) | (c) | 0.36 |
| Salt | Number of Discs | Number of Discs per Tube | Mass of Salt (kg) | Moles of Salt (kmol) | Stoichiometric Reaction Ratio |
|---|---|---|---|---|---|
| BaCl2 * | 509 | 73 | 0.787339 | 0.003781 | 8 |
| CaCl2 (4-2) | 1266 | 181 | 1.678507 | 0.015124 | 2 |
| CaCl2 (8-4) | 633 | 90 | 0.839254 | 0.007562 | 4 |
| CaCl2 (8-4-2) | 422 | 60 | 0.559502 | 0.005041 | 6 |
| MnCl2 | 501 | 72 | 0.951628 | 0.007562 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinmers, S.; Atkinson, G.H.; Critoph, R.E.; van der Pal, M. Resorption Thermal Transformer Generator Design. Energies 2022, 15, 2058. https://0-doi-org.brum.beds.ac.uk/10.3390/en15062058
Hinmers S, Atkinson GH, Critoph RE, van der Pal M. Resorption Thermal Transformer Generator Design. Energies. 2022; 15(6):2058. https://0-doi-org.brum.beds.ac.uk/10.3390/en15062058
Chicago/Turabian StyleHinmers, Samuel, George H. Atkinson, Robert E. Critoph, and Michel van der Pal. 2022. "Resorption Thermal Transformer Generator Design" Energies 15, no. 6: 2058. https://0-doi-org.brum.beds.ac.uk/10.3390/en15062058