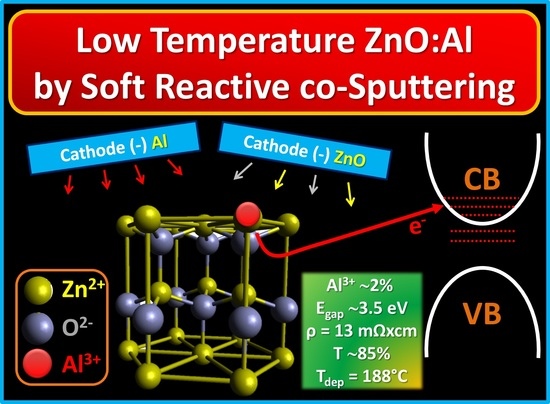
Controlled Al3+ Incorporation in the ZnO Lattice at 188 °C by Soft Reactive Co-Sputtering for Transparent Conductive Oxides
Abstract
:1. Introduction
2. Experimental Section
2.1. Deposition of the ZnO:Al Layers by Magnetron Direct Current Pulsed Sputtering
2.2. Characterization Equipments
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. Field Emission-Scanning Electron Microscopy/Energy Dispersive X-ray Analyses
3.3. X-ray Photoelectron Spectroscopy Analysis
- C 1s (at 284.7 eV);
- O 1s (at 531.4 eV);
- Zn 2p1/2 and 2p3/2 (at 1045 eV and 1022.8 eV respectively);
- Al 2p3/2 (at 74.7 eV) regions are shown in Figure 4a–d.
3.4. UV-VIS Measurements
3.5. Micro-Raman Analysis
3.6. Electrical Characterizations
4. Conclusions
- (1)
- Al3+ species effectively incorporated in the ZnO structure with negligible residual strain;
- (2)
- uniform ZnO:Al stoichiometry at least over 1 μm of grown thickness;
- (3)
- optical bandgap of ~3.5 eV;
- (4)
- ~85% transmittance in the visible range;
- (5)
- deposition rate as high as ~7 nm/min.
- (1)
- avoiding intrinsic overheating and instead using a mild controlled extrinsic heating;
- (2)
- compensating oxygen deficiency to limit the formation of oxygen defects;
- (3)
- guaranteeing a uniform incorporation of Al during growth.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TCO | Transparent conductive oxide |
| AZO | Al doped ZnO |
| ITO | Indium Tin Oxide |
| PLD | Pulsed laser deposition |
| CVD | Chemical vapor deposition |
| DC | Direct current |
| DSC | Dye-sensitized solar cells |
| XRD | X-ray diffraction |
| FE-SEM | Field emission-scanning electron microscopy |
| EDX | Energy dispersive X-ray |
| HR | High resolution |
| XPS | X-ray photoelectron spectroscopy |
| UHV | Ultra high vacuum |
| CCD | Charge-coupled detector |
| SE | Spectroscopic ellipsometry |
| BE | Binding energy |
| CB | Conduction band |
| Rs | Sheet resistance |
| sccm | standard cubic centimeter minute |
| rpm | revolutions per minute |
References
- Ko, Y.D.; Kim, J.Y.; Joung, H.C.; Ahn, S.H.; Jang, K.S.; Lee, Y.J.; Yi, J. Low temperature deposited transparent conductive ITO and IZTO films for flat panel display applications. J. Ceram. Process. Res. 2013, 14, 183–187. [Google Scholar]
- Fortunato, E.; Ginley, D.; Hosono, H.; Paine, D.C. Transparent conducting oxides for photovoltaics. MRS Bull. 2007, 32, 242–247. [Google Scholar] [CrossRef]
- Abrinaei, F.; Shirazi, M.; Hosseinnejad, M.T. Investigation of growth dynamics of nanostructured aluminum doped zinc oxide thin films deposited for the solar cell applications. J. Inorg. Organomet. Polym. 2016, 26, 233–241. [Google Scholar] [CrossRef]
- Dong, P.; Zhu, Y.; Zhang, J.; Hao, F.; Wu, J.; Lei, S.; Lin, H.; Hauge, R.H.; Tour, J.M.; Lou, J. Vertically aligned carbon nanotubes/graphene hybrid electrode as a TCO- and Pt-Free flexible cathode for application in solar cells. J. Mater. Chem. A 2014, 2, 20902–20907. [Google Scholar] [CrossRef]
- Huang, M.; Hameiri, Z.; Gong, H.; Wong, W.C.; Aberle, A.G.; Mueller, T. Novel hybrid electrode using transparent conductive oxide and silver nanoparticle mesh for silicon solar cell applications. Energy Procedia 2014, 55, 670–678. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; De Marco, L.; Giannuzzi, R.; Gigli, G.; Suranna, G.P. Molecular engineering of largely π-extended metal-free sensitizers containing benzothiadiazole units: Approaching 10% efficiency dye-sensitized solar cells using iodine-based electrolytes. Dye. Pigment. 2016, 131, 282–292. [Google Scholar] [CrossRef]
- Chou, T.P.; Zhang, Q.; Cao, G. Effects of dye loading conditions on the energy conversion efficiency of ZnO and TiO2 dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 18804–18811. [Google Scholar] [CrossRef]
- Noh, J.H.; Han, H.S.; Lee, S.; Kim, D.H.; Park, J.H.; Park, S.; Kim, J.Y.; Jung, H.S.; Hong, K.S. A newly designed Nb-doped TiO2/Al-doped ZnO transparent conducting oxide multilayer for electrochemical photoenergy conversion devices. J. Phys. Chem. C 2010, 114, 13867–13871. [Google Scholar] [CrossRef]
- Alberti, A.; De Marco, L.; Pellegrino, G.; Condorelli, G.G.; Giannuzzi, R.; Scarfiello, R.; Manca, M.; Spinella, C.; Gigli, G.; La Magna, A. A combined strategy to realize efficient photoelectrodes for low temperature fabrication of dye solar cells. ACS Appl. Mater. Interfaces 2014, 6, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Pellegrino, G.; Condorelli, G.G.; Bongiorno, C.; Morita, S.; La Magna, A.; Miyasaka, T. Efficiency enhancement in ZnO:Al-based dye-sensitized solar cells structured with sputtered TiO2 blocking layers. J. Phys. Chem. C 2014, 118, 6576–6585. [Google Scholar] [CrossRef]
- Sanzaro, S.; Pellegrino, G.; Smecca, E.; Malandrino, G.; Catalano, R.; La Magna, A.; Alberti, A. Deposition of ZnO:Al/TiO2 Bi-Layers at Low Temperature by Reactive Sputtering for Application as Photoanodes in Dye-Sensitized Solar Cells; Activity Report 2014-Dip. Di Fisica; University of Messina: Messina, Italy, 2014; pp. 87–90. [Google Scholar]
- Pellegrino, G.; Condorelli, G.G.; De Rossi, F.; Brown, T.; Giovenale, F.; Bongiorno, C.; Alberti, A. Thermally induced structural modifications of nano-sized anatase films and the effects on the dye-TiO2 surface interactions. Appl. Surf. Sci. 2014, 296, 69–78. [Google Scholar] [CrossRef]
- Chung, Y.M.; Moon, C.S.; Jung, M.J.; Han, J.G. The low temperature synthesis of Al doped ZnO films on glass and polymer using magnetron co-sputtering: Working pressure effect. Surf. Coat. Technol. 2005, 200, 936–939. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhao, L.; Lian, J.S. Al-doped ZnO films by pulsed laser deposition at room temperature. Vacuum 2006, 81, 18–21. [Google Scholar] [CrossRef]
- Hu, J.; Gordon, R.G. Textured aluminum-doped zinc oxide thin films from atmospheric pressure chemical-vapor deposition. J. Appl. Phys. 1992, 71, 880–890. [Google Scholar] [CrossRef]
- Shirahata, T.; Kawaharamura, T.; Fujita, S.; Orita, H. Transparent conductive zinc-oxide-based films grown at low temperature by mist chemical vapor deposition. Thin Solid Films 2015, 597, 30–38. [Google Scholar] [CrossRef]
- Kim, Y.S.; Tai, W.P. Electrical and optical properties of Al-doped ZnO thin films by sol-gel process. Appl. Surf. Sci. 2006, 253, 4911–4916. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhu, D.; Liu, W.; Liu, Y.; Ma, X.C. Optical properties of Al-doped ZnO thin films by ellipsometry. Appl. Surf. Sci. 2008, 254, 2922–2926. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, C.; Huang, J.; Wang, M.; He, H.; Wang, H.; Cao, Y. Effects of Al content on the properties of ZnO:Al films prepared by Al2O3 and ZnO co-sputtering. J. Mater. Sci. Mater. Electron 2010, 21, 1030–1035. [Google Scholar] [CrossRef]
- Gorgulla, A.; Ertel, D.P.; Steyer, M.; Hahn, G.; Terheiden, B. Effect of Al concentration analyzed by ICP-OES on the structural, electrical and optical properties of co-sputtered ZnO:Al films. Energy Procedia 2015, 77, 687–693. [Google Scholar] [CrossRef]
- Caramori, S.; Cristino, V.; Boaretto, R.; Argazzi, R.; Bignozzi, C.A.; Di Carlo, A. New components for dye-sensitized solar cells. Int. J. Photoenergy 2010, 2010. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Karunagaran, B.; Kim, K.; Mangalaraj, D.; Yi, J.; Velumani, S. Structural, optical and Raman scattering studies on DC magnetron sputtered titanium dioxide thin films. Solar Energy Mater. Solar Cells 2005, 88, 199–208. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous optical absorption limit in InSb. Phys. Rev. 1954, 93, 632. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoc, H.J. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Li Bassi, A. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115. [Google Scholar] [CrossRef]
- Reparaz, J.S.; Muniz, L.R.; Wagner, M.R.; Goñi, A.R.; Alonso, M.I.; Hoffmann, A.; Meyer, B.K. Reduction of the transverse effective charge of optical phonons in ZnO under pressure. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Lany, S.; Zunger, A. Anion vacancies as a source of persistent photoconductivity in II-VI and chalcopyrite semiconductors. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Lany, S.; Zunger, A. Dopability, intrinsic conductivity, and nonstoichiometry of transparent conducting oxides. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- El Manouni, A.; Manjón, F.J.; Mollar, M.; Marí, B.; Gómez, R.; López, M.C.; Ramos-Barrado, J.R. Effect of aluminium doping on zinc oxide thin films grown by spray pyrolysis. Superlattices Microstruct. 2006, 39, 185–192. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: Boston, MA, USA, 1978; p. 102. [Google Scholar]
- Clark, S.J.; Robertson, J.; Lany, S.; Zunger, A. Intrinsic defects in ZnO calculated by screened exchange and hybrid density functionals. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef]
- Maghanga, C.M.; Niklasson, G.A.; Granqvist, C.G. Optical properties of sputter deposited transparent and conducting TiO2:Nb films. Thin Solid Films 2009, 518, 1254–1258. [Google Scholar] [CrossRef]









| Sample | Intensity Ratio | Resistivity (mΩ × cm) | Conductivity (S/cm) |
|---|---|---|---|
| AZO_@138 °C | 0.38 | 125 | 8.00 |
| AZO_@155 °C | 0.44 | 90 | 11.11 |
| AZO_@163 °C | 0.36 | 69 | 14.49 |
| AZO_@171 °C | 0.28 | 71 | 14.08 |
| AZO_@188 °C | 0.18 | 13 | 76.92 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanzaro, S.; La Magna, A.; Smecca, E.; Mannino, G.; Pellegrino, G.; Fazio, E.; Neri, F.; Alberti, A. Controlled Al3+ Incorporation in the ZnO Lattice at 188 °C by Soft Reactive Co-Sputtering for Transparent Conductive Oxides. Energies 2016, 9, 433. https://0-doi-org.brum.beds.ac.uk/10.3390/en9060433
Sanzaro S, La Magna A, Smecca E, Mannino G, Pellegrino G, Fazio E, Neri F, Alberti A. Controlled Al3+ Incorporation in the ZnO Lattice at 188 °C by Soft Reactive Co-Sputtering for Transparent Conductive Oxides. Energies. 2016; 9(6):433. https://0-doi-org.brum.beds.ac.uk/10.3390/en9060433
Chicago/Turabian StyleSanzaro, Salvatore, Antonino La Magna, Emanuele Smecca, Giovanni Mannino, Giovanna Pellegrino, Enza Fazio, Fortunato Neri, and Alessandra Alberti. 2016. "Controlled Al3+ Incorporation in the ZnO Lattice at 188 °C by Soft Reactive Co-Sputtering for Transparent Conductive Oxides" Energies 9, no. 6: 433. https://0-doi-org.brum.beds.ac.uk/10.3390/en9060433