A Comparative Study on the Direct and Pulsed Current Electrodeposition of Cobalt-Substituted Hydroxyapatite for Magnetic Resonance Imaging Application
Abstract
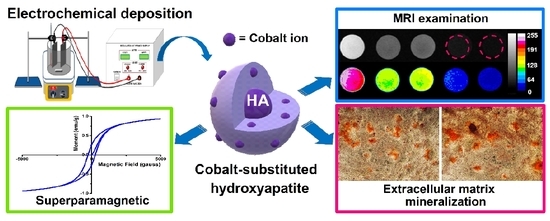
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cobalt-Substituted Hydroxyapatite by Electrochemical Deposition
2.2. Surface Characterization
2.3. Internal Ion Composition
2.4. Magnetic Analysis
2.5. In Vitro Biodegradation
2.6. Free Radical Scavenging Effects
2.7. In Vitro Magnetic Resonance Imaging (MRI) Examination
2.8. Biocompatibility
2.9. Extracellular Matrix Mineralization
2.10. Antibacterial
2.11. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
3.2. Internal Ion Composition
3.3. Magnetic Analysis
3.4. In Vitro Biodegradation
3.5. Free Radical Scavenging Effects
3.6. In Vitro MRI Examination
3.7. Biocompatibility
3.8. Extracellular Matrix Mineralization
3.9. Antibacterial
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, M.H.; Lee, P.Y.; Chen, W.C.; Hu, J.J. Comparison of three calcium phosphate bone graft substitutes from biomechanical, histological, and crystallographic perspectives using a rat posterolateral lumbar fusion model. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Dubory, A.; Pariat, J.; Potage, D.; Roubineau, F.; Jammal, S.; Lachaniette, C.F. Beta-tricalcium phosphate for orthopedic reconstructions as an alternative to autogenous bone graft. Morphologie 2017, 101, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.; Kirschner, M.; Wangemann, T.; Falk, C.; Mempel, W.; Hammer, C. Infections and immunological hazards of allogeneic bone transplantation. Arch. Orthop. Trauma Surg. 1995, 114, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, K.; Zhao, R.; Ye, X.; Chen, X.; Xiao, Z.; Yang, X.; Zhu, X.; Zhang, K.; Fan, Y.; et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials 2017, 147, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Z.; Yang, J.; Yi, Z.; Xiao, W.; Liu, X.; Yang, X.; Xu, W.; Liao, X. Preparation of bioactive beta-tricalcium phosphate microspheres as bone graft substitute materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Galea, L.; Doebelin, N. Calcium phosphate bone graft substitutes: Failures and hopes. J. Eur. Ceram. Soc. 2012, 32, 2663–2671. [Google Scholar] [CrossRef]
- Kramer, E.R.; Morey, A.M.; Staruch, M.; Suib, S.L.; Jain, M.; Budnick, J.I.; Wei, M. Synthesis and characterization of iron-substituted hydroxyapatite via a simple ion-exchange procedure. J. Mater. Sci. 2013, 48, 665–673. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Preparation and characterisation of nanophase Sr, Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater. Sci. Eng. C 2014, 35, 106–114. [Google Scholar] [CrossRef]
- Stanić, V.; Radosavljević-Mihajlović, A.S.; Živković-Radovanović, V.; Nastasijević, B.; Marinović-Cincović, M.; Marković, J.P.; Budimir, M.D. Synthesis, structural characterisation and antibacterial activity of Ag+-doped fluorapatite nanomaterials prepared by neutralization method. Appl. Surf. Sci. 2015, 337, 72–80. [Google Scholar] [CrossRef]
- Kramer, E.; Itzkowitz, E.; Wei, M. Synthesis and characterization of cobalt-substituted hydroxyapatite powders. Ceram. Int. 2014, 40, 13471–13480. [Google Scholar] [CrossRef]
- Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials 2010, 31, 3580–3589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhao, L. Hypoxia-mimicking Co doped TiO2 microporous coating on titanium with enhanced angiogenic and osteogenic activities. Acta Biomater. 2016, 43, 358–368. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram. Int. 2015, 41, 11323–11333. [Google Scholar] [CrossRef]
- Tahmasebi Birgani, Z.; Fennema, E.; Gijbels, M.J.; de Boer, J.; van Blitterswijk, C.A.; Habibovic, P. Stimulatory effect of cobalt ions incorporated into calcium phosphate coatings on neovascularization in an in vivo intramuscular model in goats. Acta Biomater. 2016, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, N.; Ajdukovic, Z.; Rajkovic, J.; Najman, S.; Mihailovic, D.; Uskokovic, D. Enhanced Osteogenesis of nanosized cobalt-substituted hydroxyapatite. J. Bionic Eng. 2015, 12, 604–612. [Google Scholar] [CrossRef]
- Sarath Chandra, V.; Elayaraja, K.; Thanigai Arul, K.; Ferraris, S.; Spriano, S.; Ferraris, M.; Asokan, K.; Narayana Kalkura, S. Synthesis of magnetic hydroxyapatite by hydrothermal–microwave technique: Dielectric, protein adsorption, blood compatibility and drug release studies. Ceram. Int. 2015, 41, 13153–13163. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y.; Wei, Z.; Qi, M. Development of multifunctional cobalt ferrite/graphene oxide nanocomposites for magnetic resonance imaging and controlled drug delivery. Chem. Eng. J. 2016, 289, 150–160. [Google Scholar] [CrossRef]
- Shokrollahi, H. Contrast agents for MRI. Mater. Sci. Eng. C 2013, 33, 4485–4497. [Google Scholar] [CrossRef]
- Stojanović, Z.; Veselinović, L.; Marković, S.; Ignjatović, N.; Uskoković, D. Hydrothermal synthesis of nanosized pure and cobalt-exchanged hydroxyapatite. Mater. Manuf. Process. 2009, 24, 1096–1103. [Google Scholar] [CrossRef]
- Veselinović, L.; Karanović, L.; Stojanović, Z.; Bračko, I.; Marković, S.; Ignjatović, N.; Uskoković, D. Crystal structure of cobalt-substituted calcium hydroxyapatite nanopowders prepared by hydrothermal processing. J. Appl. Crystallogr. 2010, 43, 320–327. [Google Scholar] [CrossRef]
- Ignjatović, N.; Ajduković, Z.; Savić, V.; Najman, S.; Mihailović, D.; Vasiljević, P.; Stojanović, Z.; Uskoković, V.; Uskoković, D. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci. Mater. Med. 2013, 24, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.; Wei, M.; Vasiliev, A.; Shaw, M. Influence of temperature and concentration on the sintering behavior and mechanical properties of hydroxyapatite. Acta Mater. 2004, 52, 5655–5663. [Google Scholar] [CrossRef]
- Isa, N.N.C.; Mohd, Y.; Yury, N. Electrochemical Deposition and Characterization of Hydroxyapatite (HAp) on Titanium Substrate. APCBEE Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Yousefpour, M.; Afshar, A.; Yang, X.; Li, X.; Yang, B.; Wu, Y.; Chen, J.; Zhang, X. Nano-crystalline growth of electrochemically deposited apatite coating on pure titanium. J. Electroanal. Chem. 2006, 589, 96–105. [Google Scholar] [CrossRef]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, D.-Y.; Oh, K.-T.; Lee, Y.-K.; Kim, K.-M.; Kim, K.-N. Bioactivity of calcium phosphate coatings prepared by electrodeposition in a modified simulated body fluid. Mater. Lett. 2006, 60, 2573–2577. [Google Scholar] [CrossRef]
- Hayakawa, T.; Kawashita, M.; Takaoaka, G.H. Coating of hydroxyapatite films on titanium substrates by electrodeposition under pulse current. J. Ceram. Soc. Jpn. 2008, 116, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Manso, M.; Jimenez, C.; Morant, C.; Herrero, P.; Martınez-Duart, J. Electrodeposition of hydroxyapatite coatings in basic conditions. Biomaterials 2000, 21, 1755–1761. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, D.-Y.; Oh, K.-T.; Lec, Y.; Kim, K.-N. Bioactive calcium phosphate coating on sodium hydroxide-pretreated titanium substrate by electrodeposition. J. Am. Ceram. Soc. 2004, 87, 1792–1794. [Google Scholar] [CrossRef]
- Dumelie, N.; Benhayoune, H.; Richard, D.; Laurent-Maquin, D.; Balossier, G. In vitro precipitation of electrodeposited calcium-deficient hydroxyapatite coatings on Ti6Al4V substrate. Mater. Charact. 2008, 59, 129–133. [Google Scholar] [CrossRef]
- Lee, K.; Jeong, Y.-H.; Ko, Y.-M.; Choe, H.-C.; Brantley, W.A. Hydroxyapatite coating on micropore-formed titanium alloy utilizing electrochemical deposition. Thin Solid Films 2013, 549, 154–158. [Google Scholar] [CrossRef]
- Marashi-Najafi, F.; Khalil-Allafi, J.; Etminanfar, M.R. Biocompatibility of hydroxyapatite coatings deposited by pulse electrodeposition technique on the Nitinol superelastic alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Yan, M.; Tang, C.-M. Effect of the electrolyte composition of cobalt substituted hydroxyapatite by electrodeposition process. In Proceedings of the 15th Asian Bioceramics Symposium (ABC2015), Tokyo, Japan, 9–11 December 2015. [Google Scholar]
- He, C.; Xiao, G.; Jin, X.; Sun, C.; Ma, P.X. Electrodeposition on nanofibrous polymer scaffolds: Rapid mineralization, tunable calcium phosphate composition and topography. Adv. Funct. Mater. 2010, 20, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Gopi, D.; Indira, J.; Kavitha, L.; Kannan, S.; Ferreira, J. Spectroscopic characterization of nanohydroxyapatite synthesized by molten salt method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 545–547. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tang, C.-M.; Tian, Y.-H.; Hsu, S.-H. Poly(vinyl alcohol) Nanocomposites Reinforced with Bamboo Charcoal Nanoparticles: Mineralization Behavior and Characterization. Materials 2015, 8, 4895–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of shape and size of cobalt ferrite nanostructures on their MRI contrast and thermal activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [PubMed]
- Gopi, D.; Karthika, A.; Nithiya, S.; Kavitha, L. In vitro biological performance of minerals substituted hydroxyapatite coating by pulsed electrodeposition method. Mater. Chem. Phys. 2014, 144, 75–85. [Google Scholar] [CrossRef]
- Utku, F.S.; Seckin, E.; Goller, G.; Tamerler, C.; Urgen, M. Carbonated hydroxyapatite deposition at physiological temperature on ordered titanium oxide nanotubes using pulsed electrochemistry. Ceram. Int. 2014, 40, 15479–15487. [Google Scholar] [CrossRef]
- Kolmas, J.; Piotrowska, U.; Kuras, M.; Kurek, E. Effect of carbonate substitution on physicochemical and biological properties of silver containing hydroxyapatites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 124–130. [Google Scholar] [CrossRef]
- Sønju Clasen, A.; Ruyter, I. Quantitative determination of type A and type B carbonate in human deciduous and permanent enamel by means of Fourier transform infrared spectrometry. Adv. Dent. Res. 1997, 11, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Ortali, C.; Julien, I.; Vandenhende, M.; Drouet, C.; Champion, E. Consolidation of bone-like apatite bioceramics by spark plasma sintering of amorphous carbonated calcium phosphate at very low temperature. J. Eur. Ceram. Soc. 2018, 38, 2098–2109. [Google Scholar] [CrossRef]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef] [Green Version]
- Gopi, D.; Indira, J.; Kavitha, L.; Sekar, M.; Mudali, U.K. Synthesis of hydroxyapatite nanoparticles by a novel ultrasonic assisted with mixed hollow sphere template method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mabilleau, G.; Filmon, R.; Petrov, P.; Baslé, M.; Sabokbar, A.; Chappard, D. Cobalt, chromium and nickel affect hydroxyapatite crystal growth in vitro. Acta Biomater. 2010, 6, 1555–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, Y.; Yin, Y.; Yao, F.; Yao, K. Modulation of nano-hydroxyapatite size via formation on chitosan–gelatin network film in situ. Biomaterials 2007, 28, 781–790. [Google Scholar] [CrossRef]
- Ding, M.; Xi, J.; Ji, Z. Size-controlled synthesis, growth mechanism and magnetic properties of cobalt microspheres. Mater. Lett. 2017, 201, 27–30. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Gu, Z.; Qin, H.; Li, L.; Liu, J.; Yu, X. In vitro study on the degradation of lithium-doped hydroxyapatite for bone tissue engineering scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 185–192. [Google Scholar] [CrossRef]
- Chen, Z.; Du, Y.; Li, Z.; Yang, K.; Lv, X. Controllable synthesis of magnetic Fe3O4 particles with different morphology by one-step hydrothermal route. J. Magn. Magn. Mater. 2017, 426, 121–125. [Google Scholar] [CrossRef]
- Sumathi, S.; Gopal, B. In vitro degradation of multisubstituted hydroxyapatite and fluorapatite in the physiological condition. J. Cryst. Growth 2015, 422, 36–43. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Fathi, M.H.; Ahangarian, M.; Zahrani, E.M. In vitro bioactivity evaluation of magnesium-substituted fluorapatite nanopowders. Ceram. Int. 2012, 38, 169–175. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Tang, C.-M.; Tseng, H.-J. Gold nanoparticles induce surface morphological transformation in polyurethane and affect the cellular response. Biomacromolecules 2007, 9, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Kim, D.K.; El Haj, A.J.; Dobson, J. Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications. IEEE Trans. Nanobiosci. 2008, 7, 298–305. [Google Scholar]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Bowers, M.E.; Tung, G.A.; Trinh, N.; Leventhal, E.; Crisco, J.J.; Kimia, B.; Fleming, B.C. Effects of ACL interference screws on articular cartilage volume and thickness measurements with 1.5 T and 3 T MRI. Osteoarthr. Cartil. 2008, 16, 572–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, Y.W.; Lee, J.H.; Cheon, J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 2008, 47, 5122–5135. [Google Scholar] [CrossRef]
- Wu, H.; Liu, G.; Wang, X.; Zhang, J.; Chen, Y.; Shi, J.; Yang, H.; Hu, H.; Yang, S. Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery. Acta Biomater. 2011, 7, 3496–3504. [Google Scholar] [CrossRef]
- Parkes, L.M.; Hodgson, R.; Lu le, T.; Tung le, D.; Robinson, I.; Fernig, D.G.; Thanh, N.T. Cobalt nanoparticles as a novel magnetic resonance contrast agent--relaxivities at 1.5 and 3 Tesla. Contrast Media Mol. Imaging 2008, 3, 150–156. [Google Scholar] [CrossRef]
- Jun, Y.-W.; Huh, Y.-M.; Choi, J.-S.; Lee, J.-H.; Song, H.-T.; Kim, S.; Kim, S.; Yoon, S.; Kim, K.-S.; Shin, J.-S. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Tanaka, O.; Nishigaki, Y.; Hayashi, H.; Iida, T.; Yokoyama, T.; Takenaka, E.; Yama, E.; Tomita, E. The advantage of iron-containing fiducial markers placed with a thin needle for radiotherapy of liver cancer in terms of visualization on MRI: An initial experience of Gold Anchor. Radiol. Case Rep. 2017, 12, 416–421. [Google Scholar] [CrossRef]
- Hossain, M.; Schirmer, T.; Richardson, T.; Chen, L.; Buyyounouski, M.K.; Ma, C.M. Effect of gold marker seeds on magnetic resonance spectroscopy of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.I.; Udrescu, C.; Enachescu, C.; Rouviere, O.; Arion, S.; Caraivan, I.; Chapet, O. Transrectal implantation and stability of gold markers in prostate bed for salvage radiotherapy of macroscopic recurrences. Phys. Med. 2016, 32, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Jazwa, A.; Wegiel, B.; Jozkowicz, A.; Dulak, J. Heme oxygenase-1-dependent and-independent regulation of angiogenic genes expression: Effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell. Mol. Boil. 2005, 51, 347. [Google Scholar]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, E.; Partap, S.; Azevedo, M.M.; Jell, G.; Stevens, M.M.; O’Brien, F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials 2015, 52, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Yuen, J.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated beta-tricalcium phosphate. Biomaterials 2015, 61, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, T.; Haider, S.; Kamal, T.; Ul-Islam, M. Thermal decomposition of metal complex precursor as route to the synthesis of Co 3 O 4 nanoparticles: Antibacterial activity and mechanism. J. Alloy Compd. 2017, 704, 296–302. [Google Scholar] [CrossRef]











| Sample | Particle Size | Magnetism Parameters | ||||
|---|---|---|---|---|---|---|
| Length (nm) | Width (nm) | Aspect Ratio | Hc (Oe) | Ms (emu/g) | Hr (emu/g) | |
| DC-CoHA | 365 ± 11 | 285 ± 80 | 1.28 | 261.41 | 0.86 | 0.26 |
| PC1-CoHA | 418 ± 80 | 370 ± 90 | 1.13 | 133.73 | 0.79 | 0.15 |
| PC2-CoHA | 602 ± 73 | 510 ± 67 | 1.18 | 132.18 | 0.65 | 0.09 |
| Sample | 2θ | Line Width (FWHM) | Crystallite Size Xs (nm) | Fraction Crystallinity Xc |
|---|---|---|---|---|
| DC-CoHA | 25.87 | 0.77 | 1.849 | 0.030 |
| PC1-CoHA | 25.94 | 1.08 | 1.318 | 0.011 |
| PC2-CoHA | 25.92 | 0.91 | 1.565 | 0.018 |
| Sample | Particle | Extraction Solution | |||
|---|---|---|---|---|---|
| Ca + Co/P | Xco (%) | Ca (ppM) | Co (ppM) | P (ppM) | |
| PBS | N.D. | N.D. | N.D. | N.D. | 541 |
| DC-CoHA | 1.72 | 14.0 | 1.04 | 1.45 | 434 |
| PC1-CoHA | 1.67 | 14.0 | 1.68 | 1.86 | 429 |
| PC2-CoHA | 1.68 | 13.0 | 1.21 | 1.72 | 425 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Chuang, C.-C.; Wang, P.-T.; Tang, C.-M. A Comparative Study on the Direct and Pulsed Current Electrodeposition of Cobalt-Substituted Hydroxyapatite for Magnetic Resonance Imaging Application. Materials 2019, 12, 116. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12010116
Lin W-C, Chuang C-C, Wang P-T, Tang C-M. A Comparative Study on the Direct and Pulsed Current Electrodeposition of Cobalt-Substituted Hydroxyapatite for Magnetic Resonance Imaging Application. Materials. 2019; 12(1):116. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12010116
Chicago/Turabian StyleLin, Wei-Chun, Chun-Chao Chuang, Pin-Ting Wang, and Cheng-Ming Tang. 2019. "A Comparative Study on the Direct and Pulsed Current Electrodeposition of Cobalt-Substituted Hydroxyapatite for Magnetic Resonance Imaging Application" Materials 12, no. 1: 116. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12010116