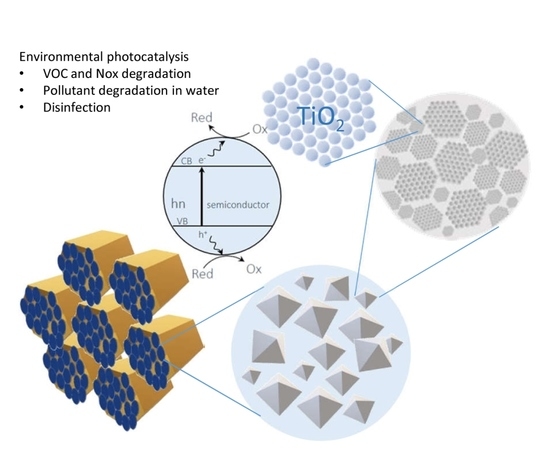
Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications
Abstract
:1. Introduction
2. Synthesis of Mesoporous TiO2
2.1. Sol-Gel Methods
2.2. Synthesis in Room Temperature Ionic Liquids
2.3. Hydrothermal Synthetic Methods
3. Environmental Applications of Mesoporous TiO2
3.1. Application for Water Treatment
3.2. Air Treatment
3.2.1. Photocatalytic Abatement of NOx by Using Mesoporous TiO2
3.2.2. Photocatalytic Abatement of Volatile Organic Compounds (VOCs) by Mesoporous TiO2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, J.; Cruz-Yusta, M.; Sánchez, L. Nanomaterials to Combat NO X Pollution. J. Nanosci. Nanotechnol. 2015, 15, 6373–6385. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.A.; Costa, P.G.; Marangoni, L.F.B.; Pereira, C.M.; Abrantes, D.P.; Calderon, E.N.; Castro, C.B.; Bianchini, A. Environmental health in southwestern Atlantic coral reefs: Geochemical, water quality and ecological indicators. Sci. Total Environ. 2018, 651, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Ferro, G.; Alferez, M.C.; Polo-López, M.I.; Fernández-Ibañez, P.; Rizzo, L. Inactivation and regrowth of multidrug resistant bacteria in urban wastewater after disinfection by solar-driven and chlorination processes. J. Photochem. Photobiol. B 2015, 148, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Petronella, F.; Diomede, S.; Fanizza, E.; Mascolo, G.; Sibillano, T.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photodegradation of nalidixic acid assisted by TiO2 nanorods/Ag nanoparticles based catalyst. Chemosphere 2013, 91, 941–947. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Sibillano, T.; Giannini, C.; Striccoli, M.; Comparelli, R.; Curri, M.L. Multifunctional TiO2/FexOy/Ag based nanocrystalline heterostructures for photocatalytic degradation of a recalcitrant pollutant. Catal. Today 2017, 284, 100–106. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Petronella, F.; Pagliarulo, A.; Striccoli, M.; Calia, A.; Lettieri, M.; Colangiuli, D.; Curri, M.L.; Comparelli, R. Colloidal Nanocrystalline Semiconductor Materials as Photocatalysts for Environmental Protection of Architectural Stone. Crystals 2017, 7, 30. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, J.; Cai, Z. TiO2 Nanoparticles in the Marine Environment: Impact on the Toxicity of Tributyltin to Abalone (Haliotis diversicolor supertexta) Embryos. Environ. Sci. Technol. 2011, 45, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.; Comparelli, R. Visible-Light-Active TiO2-Based Hybrid Nanocatalysts for Environmental Applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with Dominant High-Energy {001} Facets: Synthesis, Properties, and Applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. A critical review on the recent progress of synthesizing techniques and fabrication of TiO2-based nanotubes photocatalysts. Appl. Catal. A 2014, 481, 127–142. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV—Vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Dudem, B.; Bharat, L.K.; Leem, J.W.; Kim, D.H.; Yu, J.S. Hierarchical Ag/TiO2/Si Forest-Like Nano/Micro-Architectures as Antireflective, Plasmonic Photocatalytic, and Self-Cleaning Coatings. ACS Sustain. Chem. Eng. 2018, 6, 1580–1591. [Google Scholar] [CrossRef]
- Veziroglu, S.; Ghori, M.Z.; Kamp, M.; Kienle, L.; Rubahn, H.G.; Strunskus, T.; Fiutowski, J.; Adam, J.; Faupel, F.; Aktas, O.C. Photocatalytic Growth of Hierarchical Au Needle Clusters on Highly Active TiO2 Thin Film. Adv. Mater. Interfaces 2018, 5, 1800465. [Google Scholar] [CrossRef]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Zhang, R.; Elzatahry, A.A.; Al-Deyab, S.S.; Zhao, D. Mesoporous titania: From synthesis to application. Nano Today 2012, 7, 344–366. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, X.; Yao, Y.; Zhang, X.; Bian, G.-Q.; Wu, W.D.; Chen, X.D.; Li, W.; Selomulya, C.; Wu, Z.; et al. Scalable synthesis of wrinkled mesoporous titania microspheres with uniform large micron sizes for efficient removal of Cr(vi). J. Mater. Chem. A 2018, 6, 3954–3966. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Yoshikawa, H.; Awaga, K.; Murayama, M.; Mori, T.; Sunada, K.; Bandow, S.; Iijima, S. Preparation, Photocatalytic Activities, and Dye-Sensitized Solar-Cell Performance of Submicron-Scale TiO2 Hollow Spheres. Langmuir 2008, 24, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- Available online: https://statnano.com/ (accessed on 6 June 2019).
- Yang, H.; Coombs, N.; Sokolov, I.; Ozin, G.A. Free-standing and oriented mesoporous silica films grown at the air-water interface. Nature 1996, 381, 589–592. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, H.; Stump, A.; Ward, T.L.; Rieker, T.; Brinker, C.J. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature 1999, 398, 223. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Hao, Y.; Han, F.; Zhang, L.; Hu, L. A general method to diverse silver/mesoporous–metal–oxide nanocomposites with plasmon-enhanced photocatalytic activity. Appl. Catal. B 2015, 165, 378–388. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Nicolaou, P.A.; Shoemaker, J.A.; de la Cruz, A.A.; Dionysiou, D.D. Impact of the morphological properties of thin TiO2 photocatalytic films on the detoxification of water contaminated with the cyanotoxin, microcystin-LR. Appl. Catal. B 2009, 91, 165–173. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Camperoa, A.; Vera-Roblesb, L.I. Mesoporous TiO2 synthesis using a semi-hard biological template. Microporous Mesoporous Mater. 2018, 270, 140–148. [Google Scholar] [CrossRef]
- Yin, Q.; Xiang, J.; Wang, X.; Guo, X.; Zhang, T. Preparation of highly crystalline mesoporous TiO2 by sol–gel method combined with two-step calcining process. J. Exp. Nanosci. 2016, 11, 1127–1137. [Google Scholar] [CrossRef]
- Phattepur, H.; Siddaiah, G.B.; Ganganagappa, N. Synthesis and Characterisation of Mesoporous TiO2Nanoparticles by Novel Surfactant Assisted Sol-gel Method for the Degradation of Organic Compounds. Period. Polytech. Chem. Eng. 2018, 63, 85–95. [Google Scholar] [CrossRef]
- Ohno, T. Rutile Titanium Dioxide Nanoparticles Each Having Novel Exposed Crystal Face and Method for Producing Same. U.S. Patent Documents US 8,758,574, 24 June 2014. [Google Scholar]
- Park; Yang, S.H.; Jun, Y.-S.; Hong, W.H.; Kang, J.K. Facile Route to Synthesize Large-Mesoporous γ-Alumina by Room Temperature Ionic Liquids. Chem. Mater. 2007, 19, 535–542. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Liu, S.; Jaroniec, M. Ionic-Liquid-Assisted Synthesis of Uniform Fluorinated B/C-Codoped TiO2 Nanocrystals and Their Enhanced Visible-Light Photocatalytic Activity. Chem. Eur. J. 2013, 19, 2433–2441. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Yan, Z.; Zhu, L. Ionic Liquid-Assisted Synthesis of Large-Scale TiO2 Nanoparticles with Controllable Phase by Hydrolysis of TiCl4. ACS Nano 2009, 3, 115–122. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Synthesis of Very Small TiO2 Nanocrystals in a Room-Temperature Ionic Liquid and Their Self-Assembly toward Mesoporous Spherical Aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-T.; Wang, X.-J.; Zhao, Y.; Liu, J.-X.; Hao, Y.-J.; Liu, R.-H.; Zhao, D.-S. Ionic-liquid-assisted synthesis of high-visible-light-activated N–B–F-tri-doped mesoporous TiO2 via a microwave route. Appl. Catal. B 2014, 144, 442–453. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Hsiung, T.-L.; Wang, H.P.; Wei, Y.-L. Preparation of Nitrogen-Doped Mesoporous TiO2 with a Room-Temperature Ionic Liquid. In Proceedings of the Nanotech Conference Expo 2010, Anaheim, CA, USA, 21–24 June 2010; pp. 440–443. [Google Scholar]
- Lee, H.Y.; Kale, G.M. Hydrothermal Synthesis and Characterization of Nano-TiO2. Int. J. Appl. Ceram. Technol. 2008, 5, 657–665. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Lee, K.-H.; Song, S.-W. One-Step Hydrothermal Synthesis of Mesoporous Anatase TiO2 Microsphere and Interfacial Control for Enhanced Lithium Storage Performance. ACS Appl. Mater. Interfaces 2011, 3, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Zhu, Y.; Guo, X.; Zhou, L.; Jiang, Q. Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance. Materials 2018, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Arpaç, E.; Sayılkan, F.; Asiltürk, M.; Tatar, P.; Kiraz, N.; Sayılkan, H. Photocatalytic performance of Sn-doped and undoped TiO2 nanostructured thin films under UV and vis-lights. J. Hazard. Mater. 2007, 140, 69–74. [Google Scholar] [CrossRef]
- Querejeta, A.; Varela, A.; Parras, M.; del Monte, F.; García-Hernández, M.; González-Calbet, J.M. Hydrothermal Synthesis: A Suitable Route to Elaborate Nanomanganites. Chem. Mater. 2009, 21, 1898–1905. [Google Scholar] [CrossRef]
- Anwar, M.S.; Danish, R.; Ahmed, F.; Koo, B.H. Pressure Dependent Synthesis and Enhanced Photocatalytic Activity of TiO2 Nano-Structures. Nanosci. Nanotechnol. Lett. 2016, 8, 778–781. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Maximov, V.D.; Garshev, A.V.; Meskin, P.E.; Oleynikov, N.N.; Churagulov, B.R. Hydrothermal synthesis of nanocrystalline and mesoporous titania from aqueous complex titanyl oxalate acid solutions. Chem. Phys. Lett. 2004, 388, 411–415. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Ryoo, R.; Choi, M.; Fajula, F. Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. New J. Chem. 2003, 27, 73–79. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.-H.; Yang, F.-J. Preparation and photocatalytic activity of mesoporous TiO2 derived from hydrolysis condensation with TX-100 as template. Mater. Sci. Eng. B 2006, 128, 229–233. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwak, S.-Y. The hydrothermal synthesis of mesoporous TiO2 with high crystallinity, thermal stability, large surface area, and enhanced photocatalytic activity. Appl. Catal. A 2007, 323, 110–118. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, J.; Yu, H.; Liu, S. Low-temperature hydrothermal synthesis of highly photoactive mesoporous spherical TiO2 nanocrystalline. J. Phys. Chem. Solids 2010, 71, 507–510. [Google Scholar] [CrossRef]
- Santhosh, N.; Govindaraj, R.; Senthil Pandian, M.; Ramasamy, P.; Mukhopadhyay, S. Mesoporous TiO2 microspheres synthesized via a facile hydrothermal method for dye sensitized solar cell applications. J. Porous Mater. 2016, 23, 1483–1487. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Li, B.; Zhou, X.; Xu, N. Fabrication of Tunable Core−Shell Structured TiO2 Mesoporous Microspheres Using Linear Polymer Polyethylene Glycol as Templates. J. Phys. Chem. C 2010, 114, 2434–2439. [Google Scholar] [CrossRef]
- Ye, M.; Chen, Z.; Wang, W.; Shen, J.; Ma, J. Hydrothermal synthesis of TiO2 hollow microspheres for the photocatalytic degradation of 4-chloronitrobenzene. J. Hazard. Mater. 2010, 184, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. One-Step Fabrication and High Photocatalytic Activity of Porous TiO2 Hollow Aggregates by Using a Low-Temperature Hydrothermal Method Without Templates. Chem. Eur. J. 2007, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Sattarfard, R.; Behnajady, M.A.; Eskandarloo, H. Hydrothermal synthesis of mesoporous TiO2 nanotubes and their adsorption affinity toward Basic Violet 2. J. Porous Mater. 2018, 25, 359–371. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Asiah, M.N.; Mamat, M.H.; Khusaimi, Z.; Abdullah, S.; Rusop, M.; Qurashi, A. Surfactant-free seed-mediated large-scale synthesis of mesoporous TiO2 nanowires. Ceram. Int. 2015, 41, 4260–4266. [Google Scholar] [CrossRef]
- Guo, C.; Ge, M.; Liu, L.; Gao, G.; Feng, Y.; Wang, Y. Directed Synthesis of Mesoporous TiO2 Microspheres: Catalysts and Their Photocatalysis for Bisphenol A Degradation. Environ. Sci. Technol. 2010, 44, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Wang, X.Z.; Huang, Q.; Shen, C.; Koh, Z.Y.; Wang, Q.; Engel, A.; Bahnemann, D.W. Large-scale Synthesis of Urchin-like Mesoporous TiO2 Hollow Spheres by Targeted Etching and Their Photoelectrochemical Properties. Adv. Funct. Mater. 2014, 24, 95–104. [Google Scholar] [CrossRef]
- Baloyi, J.; Seadira, T.; Raphulu, M.; Ochieng, A. Preparation, Characterization and Growth Mechanism of Dandelion-like TiO2 Nanostructures and their Application in Photocatalysis towards Reduction of Cr(VI). Mater. Today: Proc. 2015, 2, 3973–3987. [Google Scholar] [CrossRef]
- Hu, C.; Lei, E.; Zhao, D.; Hu, K.; Cui, J.; Xiong, Q.; Liu, Z. Controllable synthesis and formation mechanism of 3D flower-like TiO2 microspheres. J. Mater. Sci.: Mater. Electron. 2018, 29, 10277–10283. [Google Scholar] [CrossRef]
- Asuha, S.; Zhou, X.G.; Zhao, S. Adsorption of methyl orange and Cr(VI) on mesoporous TiO2 prepared by hydrothermal method. J. Hazard. Mater. 2010, 181, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Song, S.; Zhang, H. Superior electrode performance of mesoporous hollow TiO2 microspheres through efficient hierarchical nanostructures. J. Power Sources 2011, 196, 8618–8624. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Khodadoust, A.P.; Kern, A.M.; Suidan, M.T.; Baudin, I.; Laîné, J.-M. Continuous-mode photocatalytic degradation of chlorinated phenols and pesticides in water using a bench-scale TiO2 rotating disk reactor. Appl. Catal. B 2000, 24, 139–155. [Google Scholar] [CrossRef]
- Ozyonar, F.; Aksoy, S.M. Removal of Salicylic Acid from Aqueous Solutions Using Various Electrodes and Different Connection Modes by Electrocoagulation. Int. J. Electrochem. Sci. 2016, 11, 3680–3696. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lützhøft, H.C.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Naskar, M.K. Sol-gel synthesis of mesoporous hollow titania microspheres for photodegradation of 4-chlorophenol. Indian J. Chem. 2018, 57A, 910–914. [Google Scholar]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Mendoza, C.; Valle, A.; Castellote, M.; Bahamonde, A.; Faraldos, M. TiO2 and TiO2–SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B 2015, 178, 155–164. [Google Scholar] [CrossRef]
- Kalousek, V.; Tschirch, J.; Bahnemann, D.; Rathouský, J. Mesoporous layers of TiO2 as highly efficient photocatalysts for the purification of air. Superlattices Microstruct. 2008, 44, 506–513. [Google Scholar] [CrossRef]
- Rathouský, J.; Kalousek, V.; Yarovyi, V.; Wark, M.; Jirkovský, J. A low-cost procedure for the preparation of mesoporous layers of TiO2 efficient in the environmental clean-up. J. Photochem. Photobiol. A-Chem. 2010, 216, 126–132. [Google Scholar] [CrossRef]
- Balbuena, J.; Calatayud, J.M.; Cruz-Yusta, M.; Pardo, P.; Martín, F.; Alarcón, J.; Sánchez, L. Mesocrystalline anatase nanoparticles synthesized using a simple hydrothermal approach with enhanced light harvesting for gas-phase reaction. Dalton Trans. 2018, 47, 6590–6597. [Google Scholar] [CrossRef]
- Qian, X.; Ren, M.; Yue, D.; Zhu, Y.; Han, Y.; Bian, Z.; Zhao, Y. Mesoporous TiO2 films coated on carbon foam based on waste polyurethane for enhanced photocatalytic oxidation of VOCs. Appl. Catal. B 2017, 212, 1–6. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Huang, H.; He, M.; Liu, S.; Liu, G.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; et al. Mesoporous TiO2 under VUV irradiation: Enhanced photocatalytic oxidation for VOCs degradation at room temperature. Chem. Eng. J. 2017, 327, 490–499. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C-Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]










| Synthetic Routes | Control Parameters | Particle Size | Pore Size | Specific Surface Area | Main Advantages | Main Drawbacks |
|---|---|---|---|---|---|---|
| Sol-gel methods [33,34,35,36,37,38,39] | pH, calcination temperature | Micrometer and sub-micrometer aggregates of nanoparticles | Pore volume from 0.18 cm3/g to 0.50 cm3/g; Pore size from 9.16 nm to 16.9 nm | From 70 m2/g to 150 m2/g | Low cost; User-friendly protocol Special facilities not required | Difficult control on the morphological and textural properties; calcination step required |
| Synthesis in room temperature ionic liquids [41,42,43,44,45,46] | Viscosity, temperature, stirring | Crystallite size 3–6 nm | Pore size 5 nm to 8 nm | 554 m2/g | Low temperature required | High cost due to the use of ionic liquid |
| Hydrothermal synthesis [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | pH, temperature, pressure, filling volume, hydrothermal treatment duration | Micrometric particles formed by nanoparticles from 3.4 nm to 27 nm | Pore volume from 0.18 cm3/g to 0.50 cm3/g Pore size from 3.4 nm to 27 nm | From 25 m2/g to 395 m2/g | High control over morphology and textural properties; Use of aqueous suspensions; Calcination step not always required: Facile photocatalyst recovering | Use of a specific facility (autoclave) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronella, F.; Truppi, A.; Dell’Edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications. Materials 2019, 12, 1853. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12111853
Petronella F, Truppi A, Dell’Edera M, Agostiano A, Curri ML, Comparelli R. Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications. Materials. 2019; 12(11):1853. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12111853
Chicago/Turabian StylePetronella, Francesca, Alessandra Truppi, Massimo Dell’Edera, Angela Agostiano, M. Lucia Curri, and Roberto Comparelli. 2019. "Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications" Materials 12, no. 11: 1853. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12111853