Chemical Modification of Poly(1-Trimethylsylil-1-Propyne) for the Creation of Highly Efficient CO2-Selective Membrane Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PTMSP on TaCl5–TIBA
2.3. Synthesis of Brominated PTMSP
2.4. Quaternization of N-Butylimidazole by the Brominated PTMSP
2.5. Physico-Chemical Characterization
3. Results and Discussions
3.1. Synthesis and Properties of PTMSP Containing Butylimidazole Bromide
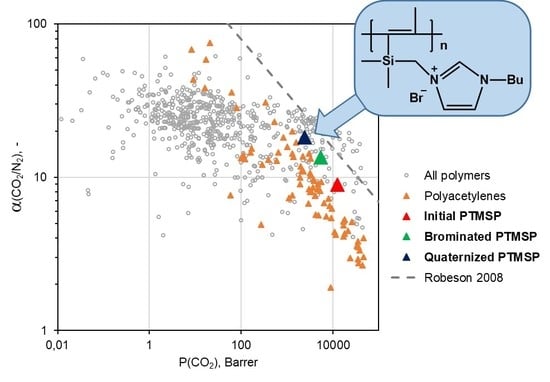
3.2. Gas Transport Characteristics of PTMSP Containing Butylimidazole Salts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tome, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Hilliard, M.; Rochelle, G. Amine volatility in CO2 capture. Int. J. Greenh. Gas Control 2010, 4, 707–715. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Ünveren, E.; Monkul, B.; Sarıoglan, S.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A Review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. A review on common adsorbents for acid gases removal: Focus on biochar. Renew. Sust. Energ. Rew. 2018, 81, 1705–1720. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.N.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Hart, A.; Gnanendran, N. Cryogenic CO2 Capture in Natural Gas. Energy Proc. 2009, 1, 697–706. [Google Scholar] [CrossRef]
- Low, B.T.; Zhao, L.; Merkel, T.C.; Weber, M.; Stolten, D. A parametric study of the impact of membrane materials and process operating conditions on carbon capture from humidified flue gas. J. Membr. Sci. 2013, 431, 139–155. [Google Scholar] [CrossRef]
- Maas, P.; Nauels, N.; Zhao, L.; Markewitz, P.; Scherer, V.; Modigell, M.; Stolten, D.; Hake, J.-F. Energetic and economic evaluation of membrane-based carbon capture routes for power plant processes. Int. J. Greenh. Gas. Control 2016, 44, 124–139. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Shahrom, M.S.R.; Wilfred, C.D.; Taha, A.K.Z. CO2 capture by task specific ionic liquids (TSILs) and polymerized ionic liquids (PILs and AAPILs). J. Mol. Liq. 2016, 219, 306–312. [Google Scholar] [CrossRef]
- Bara, J.E.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.L.; Noble, R.D. Guide to CO2 Separations in Imidazolium-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Env. 2016, 1, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Ito, H.; Masuda, T.; Hashimoto, T. Highly CO2-permeable and -permselective poly(diphenylacetylene)s having imidazolium salts: Synthesis, characterization, gas permeation properties, and effects of counter anion. Polymer 2013, 54, 6709–6715. [Google Scholar] [CrossRef]
- Polevaya, V.G.; Vorobei, A.M.; Pokrovskiy, O.I.; Shandryuk, G.A.; Parenago, O.O.; Lunin, V.V.; Khotimskiy, V.S. Modification of Poly(4-Methyl-2-Pentyne) in Supercritical Fluid Medium for Production of CO2-Selective Gas-Separation Membranes. J. Phys. Chem. B. 2017, 11, 1276–1282. [Google Scholar] [CrossRef]
- Khotimsky, V.; Tchirkova, M.; Litvinova, E.; Rebrov, A.; Bondarenko, G. Poly [1-(trimethylgermyl)-1-propyne] and Poly [1-(trimethylsilyl)-1-propyne] with Various Geometries: Their Synthesis and Properties. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2133–2155. [Google Scholar] [CrossRef]
- Polevaya, V.G.; Bondarenko, G.N.; Shandryuk, G.A.; Dolzhikova, V.D.; Khotimskiy, V.S. Synthesis and properties of brominated poly(1-trimethylsilyl-1-propyne). Russ. Chem. Bull. 2016, 65, 1067–1071. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Sultanov, E.Y.; Ezhov, A.A.; Shishatskiy, S.M.; Buhr, K.; Khotimskiy, V.S. Synthesis, Characterization, and Properties of Poly (1-trimethylsilyl-1-propyne)-block-poly(4-methyl-2-pentyne) Block Copolymers. Macromolecules 2012, 45, 1222–1229. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of Poly (ethylene glycol) Segment Length on CO2 Permeation and Stability of PolyActive Membranes and Their Nanocomposites with PEG POSS. Appl. Mater. Interfaces 2015, 23, 12289–12298. [Google Scholar] [CrossRef] [PubMed]
- Shishatskii, S.M.; Yampol’skii, Y.P.; Peinemann, K.-V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, P.; Zhang, S.; Yao, Z.; Luo, X.; Ye, L.J. Study on mechanism and ability of removal thiol from methyl tert-butyl ether by ionic liquid [BIm]Cl/CuCl. Fuel Chem. Technol. 2015, 43, 1113–1119. [Google Scholar] [CrossRef]
- Legkov, S.A.; Bondarenko, G.N.; Kostina, J.V. Structural Features of Disubstituted Polyacetylenes with Bulky Substituents at Double Bonds. Polym. Sci. Ser. A 2012, 54, 187–194. [Google Scholar] [CrossRef]
- Plate, N.A.; Khotimskiy, V.S.; Teplyakov, V.V.; Antipov, E.M.; Yampolskiy, Y.P. Polysilicon hydrocarbons: Synthesis, structure and gas separating properties: Review. Polym. Sci. USSR 1990, 32, 1053–1068. [Google Scholar] [CrossRef]
- Ovchinnikov, Y.K.; Antipov, E.M.; Markova, G.S.; Bakeev, N.F. Comparative investigation of short-range order in unbranched alkanes and polyethylene. Makromol. Chem. 1976, 177, 1567–1581. [Google Scholar] [CrossRef]
- Polikarpov, V.M.; Antipov, E.E.; Razumovskaya, I.V.; Bryantseva, I.S.; Litvinova, E.G.; Chirkova, M.V.; Korolev, Y.M.; Khotimskii, V.S.; Antipov, E.M. Comparative Study of the Structure of Membrane Si- and Ge-Containing Polymers. Polym. Sci. Ser. A 2002, 44, 343–351. [Google Scholar]
- Shishatskiy, S.; Pauls, J.R.; Nunes, S.P.; Peinemann, K.-V. Quaternary ammonium membrane materials for CO2 separation. J. Membr. Sci. 2010, 359, 44–53. [Google Scholar] [CrossRef]
- Membrane Society of Australasia. Polymer Gas Separation Membrane Database. Available online: https://membrane-australasia.org/member-portal/polymer-gas-separation-membrane-database/ (accessed on 15 July 2019).






| [Br]/[N-Butylimidazole] [mol/mol] | Content of N in the Polymer [wt%] | Content of Quaternized Units in the Polymer [mol%] |
|---|---|---|
| 1:2 | 0.4 | 2.5 |
| 1:5 | 0.8 | 5.0 |
| 1:8 | 1.7 | 10.0 |
| 1:10 | 3.1 | 20.0 |
| Content of Quaternized Units in the Polymer [mol%] | THF | CHCl3 | Toluene, Benzene | CCl4 | Cyclohexane | C5–C12 3 |
|---|---|---|---|---|---|---|
| 0 2 | + | + | + | + | + | – |
| 2.5 | + | + | + | – | – | – |
| 5 | + | + | – | – | – | – |
| 10 | ± | ± | – | – | – | – |
| 20 | – | – | – | – | – | – |
| Content of Quaternized Units in the Polymer [mol%] | 2θ [°], Basic Reflex | Δ1/2 [°] | Interplanar Distance d [Å] |
|---|---|---|---|
| 0 1 | 9.8 | 3.2 | 9.0 |
| 0 2 | 9.3 | 3.0 | 9.5 |
| 5 | 9.6 | 3.7 | 9.3 |
| 20 | 9.3 | 3.4 | 9.5 |
| Content of Quaternized Units in the Polymer [mol%] | P [Barrer] 1 | D × 107 [cm2/s] | S × 103 [cm3(STP)/ (cm3 × cmHg)] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O2 | N2 | CO2 | CH4 | O2 | N2 | CO2 | CH4 | O2 | N2 | CO2 | CH4 | |
| 0 2 | 2640 | 1400 | 12,500 | 2880 | 55 | 35 | 44 | 34 | 48 | 40 | 284 | 85 |
| 2.5 | 1200 | 406 | 5480 | 836 | 50 | 27 | 28 | 19 | 24 | 15 | 197 | 44 |
| 5 | 429 | 133 | 2410 | 336 | 39 | 19 | 13 | 16 | 11 | 7 | 185 | 21 |
| Content of Quaternized Units in the Polymer [mol%] | O2/N2 | CO2/N2 | CO2/CH4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| αP | αD | αS | αP | αD | αS | αP | αD | αS | |
| 0 1 | 1.9 | 1.6 | 1.2 | 8.8 | 1.3 | 7.1 | 4.4 | 1.3 | 3.3 |
| 2.5 | 3.0 | 1.9 | 1.6 | 13.5 | 1.0 | 13.1 | 6.6 | 1.5 | 4.5 |
| 5 | 3.2 | 2.1 | 1.6 | 18.1 | 0.7 | 26.4 | 7.2 | 0.8 | 8.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polevaya, V.; Geiger, V.; Bondarenko, G.; Shishatskiy, S.; Khotimskiy, V. Chemical Modification of Poly(1-Trimethylsylil-1-Propyne) for the Creation of Highly Efficient CO2-Selective Membrane Materials. Materials 2019, 12, 2763. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12172763
Polevaya V, Geiger V, Bondarenko G, Shishatskiy S, Khotimskiy V. Chemical Modification of Poly(1-Trimethylsylil-1-Propyne) for the Creation of Highly Efficient CO2-Selective Membrane Materials. Materials. 2019; 12(17):2763. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12172763
Chicago/Turabian StylePolevaya, Viktoriya, Viktoriya Geiger, Galina Bondarenko, Sergey Shishatskiy, and Valeriy Khotimskiy. 2019. "Chemical Modification of Poly(1-Trimethylsylil-1-Propyne) for the Creation of Highly Efficient CO2-Selective Membrane Materials" Materials 12, no. 17: 2763. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12172763