The Influence of the Electrodeposition Parameters on the Properties of Mn-Co-Based Nanofilms as Anode Materials for Alkaline Electrolysers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Electrocatalysts
2.3. Electrochemical Measurements
2.4. Material Characterisation
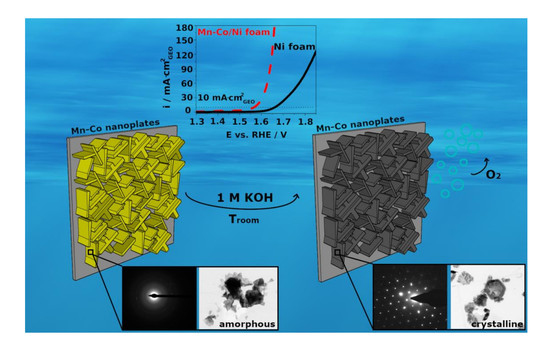
3. Results and Discussion
3.1. Electrochemical Formation and Morphology of Mn-Co-Based Oxides/Hydroxides on Ni Foam
3.2. XRD, XPS, and TEM Analysis of Mn-Co-Based Nanofilm
3.3. Probable Synthesis Mechanism for the Electrodeposition of Mn-Co Film on Nickel
3.4. OER Performance of Mn-Co/Ni-Based Electrocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Bodner, M.; Hofer, A.; Hacker, V. H2 generation from alkaline electrolyzer. Wiley Interdiscip. Rev. Energy Environ. 2015, 4, 365–381. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y. Ni Foam-supported Fe-Doped β-Ni(OH)2 nanosheets show ultralow overpotential for oxygen evolution reaction. ACS Energy Lett. 2019, 4, 622–628. [Google Scholar] [CrossRef]
- Mitra, D.; Trinh, P.; Malkhandi, S.; Mecklenburg, M.; Heald, S.M.; Balasubramanian, M.; Narayanan, S.R. An efficient and robust surface-modified iron electrode for oxygen evolution in alkaline water electrolysis. J. Electrochem. Soc. 2018, 165, F392–F400. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-Precious electrodes for practical alkaline water electrolysis. Materials 2019, 12, 1336. [Google Scholar] [CrossRef] [Green Version]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Li, J.; Zheng, G. One-dimensional earth-abundant nanomaterials for water-splitting electrocatalysts. Adv. Sci. 2017, 4, 1600380. [Google Scholar] [CrossRef]
- Shen, C.; Xu, H.; Liu, L.; Hu, H.; Chen, S.; Su, L.; Wang, L. Facile One-step dynamic hydrothermal synthesis of spinel limn2o4/carbon nanotubes composite as cathode material for lithium-ion batteries. Materials 2019, 12, 4123. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, H.; Xu, P.; Wang, R.; Tong, Y.; Lu, Q.; Gao, F. In situ construction of hierarchical Co/MnO@graphite carbon composites for highly supercapacitive and OER electrocatalytic performances. Nanoscale 2018, 10, 13702–13712. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Sun, W.; Wang, L.; Yan, Q. 2D Transition metal Oxides/Hydroxides for energy-storage applications. Chem. Nano. Mat. 2016, 2, 562–577. [Google Scholar] [CrossRef]
- Nguyen, T.; Boudard, M.; Carmezim, M.J.; Montemor, M.F. Layered Ni(OH)2-Co(OH)2 films prepared by electrodeposition as charge storage electrodes for hybrid supercapacitors. Sci. Rep. 2017, 7, 39980. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: An application-inspired renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Sahraie, N.R.; Bergmann, A.; Strasser, P.; Driess, M. Cobalt-manganese-based spinels as multifunctional materials that unify catalytic water oxidation and oxygen reduction reactions. ChemSusChem 2015, 8, 164–167. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.-B.; Lu, C.; Liu, M.-C.; Luo, Y.-C.; Kang, L.; Li, X.; Walsh, F.C. The specific capacitance of sol–gel synthesised spinel MnCo2O4 in an alkaline electrolyte. Electrochim. Acta 2014, 115, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-C.; Kong, L.-B.; Lu, C.; Li, X.-M.; Luo, Y.-C.; Kang, L. A Sol–gel process for fabrication of NiO/NiCo2O4Co3O4 composite with improved electrochemical behavior for electrochemical capacitors. ACS Appl. Mater. Interfaces 2012, 4, 4631–4636. [Google Scholar] [CrossRef]
- Zhang, Y.; Xuan, H.; Xu, Y.; Guo, B.; Li, H.; Kang, L.; Han, P.; Wang, D.; Du, Y. One-step large scale combustion synthesis mesoporous MnO2/MnCo2O4 composite as electrode material for high-performance supercapacitors. Electrochim. Acta 2016, 206, 278–290. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, C.; Wu, L.; Lang, X.; Liu, K.; Hu, Z. 3D network-like porous MnCo2O4 by the sucrose-assisted combustion method for high-performance supercapacitors. Ceram. Int. 2017, 43, 1968–1974. [Google Scholar] [CrossRef]
- Yuan, Y.; He, G.; Zhu, J. A Facile Hydrothermal Synthesis of a MnCo2O4 reduced graphene oxide nanocomposite for applicationin supercapacitors. Chem. Lett. 2014, 43, 83–85. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Li, Q.; Yu, X.; Hong, Z.; Zhang, X.; Liang, C.; Lin, Z. Hierarchical NiCo2O4 hollow microcuboids as bifunctional electrocatalysts for overall water-splitting. Angew. Chem. 2016, 128, 6398–6402. [Google Scholar] [CrossRef]
- Ma, W.; Ma, R.; Wang, C.; Liang, J.; Liu, X.; Zhou, K.; Sasaki, T. A superlattice of alternately stacked Ni-Fe hydroxide nanosheets and graphene for efficient splitting of water. ACS Nano 2015, 9, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Guo, D.; Kang, J.; Liu, L.; Zhu, C.; Gao, P.; Zhang, X.; Chen, Y. Fast fabrication of ultrathin CoMn LDH nanoarray as flexible electrode for water oxidation. Electrochim. Acta 2018, 283, 755–763. [Google Scholar] [CrossRef]
- Li, G.; Yang, D.; Chuang, P.-Y. Defining nafion ionomer roles for enhancing alkaline oxygen evolution electrocatalysis. ACS Catal. 2018, 8, 11688–11698. [Google Scholar] [CrossRef]
- Sahoo, S.; Naik, K.K.; Rout, C.S. Electrodeposition of spinel MnCo2O4 nanosheets for supercapacitor applications. Nanotechnology 2015, 26, 455401. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Ultrathin nanoflakes of cobalt-manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Wu, L.K.; Hu, J.M. A silica co-electrodeposition route to nanoporous Co3O4 film electrode for oxygen evolution reaction. Electrochim. Acta 2014, 116, 158–163. [Google Scholar] [CrossRef]
- Nguyen, T.; Boudard, M.; Rapenne, L.; Chaix-Pluchery, O.; Carmezim, M.J.; Montemor, M.F. Structural evolution, magnetic properties and electrochemical response of MnCo2O4 nanosheet films. RSC Adv. 2015, 5, 27844–27852. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, X.; MacFarlane, D.R. Dual-MnCo2O4/Ni electrode with three-level hierarchy for high-performance electrochemical energy storage. Electrochim. Acta 2018, 280, 55–61. [Google Scholar] [CrossRef]
- Pan, G.T.; Chong, S.; Yang, T.C.K.; Huang, C.M. Electrodeposited porous Mn1.5Co1.5O4/Ni composite electrodes for high-voltage asymmetric supercapacitors. Materials 2017, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Ivey, D.G. Nucleation and growth of electrodeposited Mn oxide rods for supercapacitor electrodes. Nanotechnology 2015, 26, 384001. [Google Scholar] [CrossRef]
- Vigil, J.A.; Lambert, T.N.; Eldred, K. Electrodeposited MnOx/PEDOT composite thin films for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 22745–22750. [Google Scholar] [CrossRef] [PubMed]
- Maile, N.C.; Fulari, V. Electrochemical synthesis of Mn(OH)2 and Co(OH)2 thin films for supercapacitor electrode application. Aarhat Multidiscip. Int. Educ. Res. J. 2018, 48178, 48818. [Google Scholar]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Castro, E.B.; Gervasi, C.A.; Vilche, J.R. Oxygen evolution on electrodeposited cobalt oxides. J. Appl. Electrochem. 1998, 28, 835–841. [Google Scholar] [CrossRef]
- Pérez-Alonso, F.J.; Adán, C.; Rojas, S.; Peña, M.A.; Fierro, J.L.G. Ni/Fe electrodes prepared by electrodeposition method over different substrates for oxygen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2014, 39, 5204–5212. [Google Scholar] [CrossRef]
- Han, S.; Liu, S.; Wang, R.; Liu, X.; Bai, L.; He, Z. One-Step Electrodeposition of Nanocrystalline ZnxCo3-xO8 Films with High Activity and Stability for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 17186–17194. [Google Scholar] [CrossRef]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Davis, D.J.; Limmer, S.J.; Burton, P.D.; Coker, E.N.; Beechem, T.E.; Brumbach, M.T. Electrodeposited NixCo3 nanostructured films as bifunctional oxygen electrocatalysts. Chem. Commun. 2015, 51, 9511–9514. [Google Scholar] [CrossRef]
- Koza, J.A.; He, Z.; Miller, A.S.; Switzer, J.A. Electrodeposition of crystalline Co3O4-A catalyst for the oxygen evolution reaction. Chem. Mater. 2012, 24, 3567–3573. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zheng, L.R.; Zhang, B.; Yang, H.G. Electrochemical etching of α-cobalt hydroxide for improvement of oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 9578–9584. [Google Scholar] [CrossRef]
- Castro, E.B.; Gervasi, C.A. Electrodeposited Ni-Co-oxide electrodes: Characterization and kinetics of the oxygen evolution reaction. Int. J. Hydrogen Energy 2000, 25, 1163–1170. [Google Scholar] [CrossRef]
- Kim, T.W.; Woo, M.A.; Regis, M.; Choi, K.S. Electrochemical synthesis of spinel type ZnCo2O4 electrodes for use as oxygen evolution reaction catalysts. J. Phys. Chem. Lett. 2014, 5, 2370–2374. [Google Scholar] [CrossRef]
- Bao, J.; Xie, J.; Lei, F.; Wang, Z.; Liu, W.; Xu, L.; Guan, M.; Zhao, Y.; Li, H. Two-dimensional Mn-Co LDH/Graphene composite towards high-performance water splitting. Catalysts 2018, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Hu, Y.; Qian, Q.; Yao, Y.; Zhang, S.; Li, Z.; Zou, Z. Formation of Hierarchical structure composed of (Co/Ni)Mn-LDH nanosheets on MWCNT backbones for efficient electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef] [PubMed]
- Lankauf, K.; Cysewska, K.; Karczewski, J.; Mielewczyk-Gryn, A.; Górnicka, K.; Cempura, G.; Chen, M.; Jasiński, P.; Molin, S. MnxCo3-xO4 spinel oxides as efficient oxygen evolution reaction catalysts in alkaline media. Int. J. Hydrogen Energy 2020, 45, 14867–14879. [Google Scholar] [CrossRef]
- Qiao, Y.; Jiang, K.; Deng, H.; Zhou, H. A high-energy-density and long-life lithium-ion battery via reversible oxide–peroxide conversion. Nat. Catal. 2019, 2, 1035–1044. [Google Scholar] [CrossRef]
- Grdeń, M.; Alsabet, M.; Jerkiewicz, G. Surface science and electrochemical analysis of nickel foams. ACS Appl. Mater. Interfaces 2012, 4, 3012–3021. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhong, Y.; Zhou, W.; Shao, Z. Facile synthesis of nitrogen-doped carbon nanotubes encapsulating nickel cobalt alloys 3D networks for oxygen evolution reaction in an alkaline solution. J. Power Sources 2017, 338, 26–33. [Google Scholar] [CrossRef]
- Olivier, J.P.; Conklin, W.B.; Szombathely, M.V. Determination of pore size distribution from density functional theory: A comparison of nitrogen and argon results. Stud. Surf. Sci. Catal. 1994, 87, 81–89. [Google Scholar] [CrossRef]
- Liu, S.; Lee, S.C.; Patil, U.; Shackery, I.; Kang, S.; Zhang, K.; Park, J.H.; Chung, K.Y.; Chan Jun, S. Hierarchical MnCo-layered double hydroxides@Ni(OH)2 core–shell heterostructures as advanced electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 1043–1049. [Google Scholar] [CrossRef]
- Li, R.; Hu, Z.; Shao, X.; Cheng, P.; Li, S.; Yu, W.; Lin, W.; Yuan, D. Large scale synthesis of NiCo layered double hydroxides for superior asymmetric electrochemical capacitor. Sci. Rep. 2016, 6, 18737. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Takada, K.; Sasaki, T. Selective and controlled synthesis of α- and β-cobalt hydroxides in highly developed hexagonal platelets. J. Am. Chem. Soc. 2005, 127, 13869–13874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Niu, C.; Zhang, L.; Guo, H.; Wen, X.; Liang, C.; Zeng, G. Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate. Chemosphere 2018, 204, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Fujiwara, M.; Matsushita, T.; Ikeda, S. Evaluation of Mn3s X-ray photoelectron spectroscopy for characterization of manganese complexes. J. Electron Spectrosc. Relat. Phenom. 1995. [Google Scholar] [CrossRef]
- Luo, X.; Lee, W.-T.; Xing, G.; Bao, N.; Yonis, A.; Chu, D.; Lee, J.; Ding, J.; Li, S.; Yi, J. Ferromagnetic ordering in Mn-doped ZnO nanoparticles. Nanoscale Res. Lett. 2014, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Low, J.; Liu, T.; Yu, J.; Cao, S. Hierarchical hollow cages of Mn-Co layered double hydroxide as supercapacitor electrode materials. Appl. Surf. Sci. 2017, 413, 35–40. [Google Scholar] [CrossRef]
- Cysewska, K.; Karczewski, J.; Jasiński, P. Influence of electropolymerization conditions on the morphological and electrical properties of PEDOT film. Electrochim. Acta 2015, 176, 156–161. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, C.; Li, C.; Zhang, S.; Zhang, X.; Chen, Y. Highly stable three-dimensional nickel–iron oxyhydroxide catalysts for oxygen evolution reaction at high current densities. Electrochim. Acta 2017, 245, 770–779. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Zhu, Q.; Sun, J.; Qin, F.; Yu, L.; Ren, Z. Water splitting by electrolysis at high current densities under 1.6 volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.-Y.; Liu, Z.; Li, Z.; Lv, L.; Ao, X.; Tian, Y.; Zhang, Y.; Jiang, J.; Wang, C. Cuju-structured iron diselenide-derived oxide: A highly efficient electrocatalyst for water oxidation. ACS Appl. Mater. Interfaces 2017, 9, 40351–40359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.; Liu, S.; Zhuang, X.; Chen, M.; Zschech, E.; Feng, X. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437. [Google Scholar] [CrossRef]
- Mefford, J.T.; Akbashev, A.R.; Zhang, L.; Chueh, W.C. Electrochemical reactivity of faceted β-Co(OH)2 single crystal platelet particles in alkaline electrolytes. J. Phys. Chem. C 2019, 123, 18783–18794. [Google Scholar] [CrossRef]
- Park, K.R.; Jeon, J.E.; Ali, G.; Ko, Y.-H.; Lee, J.; Han, H.; Mhin, S. Oxygen evolution reaction of Co-Mn-O electrocatalyst prepared by solution combustion synthesis. Catalysts 2019, 9, 564. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zheng, H.; Lu, G.; Wang, P.; Xing, L.; Wang, J.; Wang, G. Enhanced water splitting electrocatalysis over mnco2o4 via introduction of suitable ce content. ACS Sustain. Chem. Eng. 2019, 7, 1169–1177. [Google Scholar] [CrossRef]







| Element | As-Deposited Film | Film after Alkaline Treatment |
|---|---|---|
| Co2+ | 79% | 50% |
| Co3+ | 21% | 50% |
| Mn2+ | 100% | Difficult to determine |
| Mn3+ | 0% | Difficult to determine |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cysewska, K.; Rybarczyk, M.K.; Cempura, G.; Karczewski, J.; Łapiński, M.; Jasinski, P.; Molin, S. The Influence of the Electrodeposition Parameters on the Properties of Mn-Co-Based Nanofilms as Anode Materials for Alkaline Electrolysers. Materials 2020, 13, 2662. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13112662
Cysewska K, Rybarczyk MK, Cempura G, Karczewski J, Łapiński M, Jasinski P, Molin S. The Influence of the Electrodeposition Parameters on the Properties of Mn-Co-Based Nanofilms as Anode Materials for Alkaline Electrolysers. Materials. 2020; 13(11):2662. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13112662
Chicago/Turabian StyleCysewska, Karolina, Maria Krystyna Rybarczyk, Grzegorz Cempura, Jakub Karczewski, Marcin Łapiński, Piotr Jasinski, and Sebastian Molin. 2020. "The Influence of the Electrodeposition Parameters on the Properties of Mn-Co-Based Nanofilms as Anode Materials for Alkaline Electrolysers" Materials 13, no. 11: 2662. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13112662