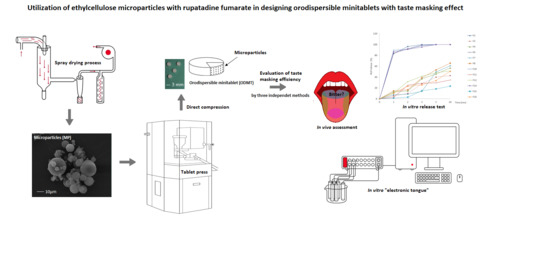
Utilization of Ethylcellulose Microparticles with Rupatadine Fumarate in Designing Orodispersible Minitablets with Taste Masking Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ODMT
2.3. Flow Properties of Powders
2.4. Evaluation of Morphology of ODMT
2.5. Quality Assessment of ODMT
2.5.1. Uniformity of Weight and Thickness
2.5.2. Mechanical Properties
2.5.3. Drug Content
2.6. Disintegration Time Assessment
2.6.1. In Vivo
2.6.2. Petri Dish
2.6.3. Texture Analyzer
2.6.4. Wettability
2.6.5. Differential Scanning Calorimetry
2.7. Evaluation of Taste Masking Effectiveness
2.7.1. In Vivo
2.7.2. RUP Dissolution
2.7.3. Electronic Tongue
Reagents and Membrane Materials
Membrane Preparation
- (1)
- weighing inner layer components: 30% (w/w) PVC, 70% (w/w) of plasticizers, DOS or o-NPOE,
- (2)
- mixing and deaerating of obtained mixture,
- (3)
- filling the Teflon holder with mixture to cover the silver-silver chloride electrode,
- (4)
- gelating inner layer (1) at 373 K for 30 min, cooling of the gelled layer,
- (5)
- weighing outer layer components, 27–33% (w/w) of PVC, 64–68% (w/w) of plasticizers, 1–5% of electroactive components (2) (Table 2),
- (6)
- dissolving of obtained mixture in THF,
- (7)
- placing drops on the inner layer (1),
- (8)
- gelating outer layer (2) in result of evaporation THF at 293 K; repeating the steps several times.
Potentiometric Measurements
Data Analysis
3. Results and Discussion
3.1. Pharmaceutical Evaluation of ODMT
3.2. Taste-Masking Efficiency Evaluation
3.2.1. In Vivo Taste Evaluation
3.2.2. In Vitro RUP Release
3.2.3. Electronic Tongue
3.2.4. Electronic Tongue—Taste Evaluation
3.2.5. Electronic Tongue—Prediction of Dissolution Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gore, R.; Chugh, P.K.; Tripathi, C.D.; Lhamo, Y.; Gautam, S. Paediatric off-label and unlicensed drug use and its implications. Curr. Clin. Pharmacol. 2017, 12, 18–25. [Google Scholar] [CrossRef]
- McIntyre, J.; Conroy, S.; Avery, A.; Corns, H.; Choonara, I. Unlicensed and of label prescribing of drugs in general practice. Arch. Dis. Child. 2000, 83, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to paediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. J. Pediatr. 2013, 163, 1728–1732. [Google Scholar] [CrossRef]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of uncoated mini-tablets in neonates-a randomized controlled trial. J. Pediatr. 2015, 167, 893–896. [Google Scholar] [CrossRef]
- Kluk, A.; Sznitowska, M.; Brandt, A.; Sznurkowska, K.; Plata-Nazar, K.; Mysliwiec, M.; Kaminska, B.; Kotłowska, H. Can preschool-aged children swallow several minitablets at a time? Results from a clinical pilot study. Int. J. Pharm. 2015, 485, 1–6. [Google Scholar] [CrossRef]
- Kumar, K.P.; Teotia, D. A comprehensieve review on pharmaceutical minitablets. J. Drug Deliv. Ther. 2018, 8, 382–390. [Google Scholar]
- Aleksovski, A.; Dreu, R.; Gašperlin, M.; Planinšek, O. Mini-tablets: A contemporary system for oral drug delivery in targeted patient group. Expert Opin. Drug Deliv. 2015, 12, 65–84. [Google Scholar] [CrossRef]
- Guidance for Industry. Orally Disintegrating Tablets. Available online: https://www.fda.gov/media/70877/download (accessed on 20 February 2020).
- Sharma, S.; Lewis, S. Taste masking technologies: A review. Int. J. Pharm. Sci. 2010, 2, 1–8. [Google Scholar]
- Pandey, S.; Kumar, S.; Prajapati, S.K.; Madhar, N.M. An overview on taste physiology and masking of bitter drugs. Int. J. Pharm. Bio. Sci. 2010, 1, 1–11. [Google Scholar]
- Jyothi, N.V.N.; Sakarkar, S.N.; Kumar, G.Y.; Prasanna, M. Microencapsulation techniques, factors influencing encapsulation efficiency: A review. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.D.; Selomuyla, C. On the spray drying of uniform functional microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Wasilewska, K.; Szekalska, M.; Ciosek-Skibinska, P.; Lenik, J.; Basa, A.; Jacyna, J.; Markuszewski, M.; Winnicka, K. Ethylcellulose in organic solution or aqueous dispersion form in designing taste-masked microparticles by the spray drying technique with a model bitter drug: Rupatadine fumarate. Polymers 2019, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Bowles, B.J.; Dziemidowicz, K.; Lopez, F.L.; Orlu, M.; Tuleu, C.; Edwards, A.; Ernest, T.B. Co-processed excipients for dispersible tablets—Part 1: Manufacturability. AAPS PharmSciTech 2018, 19, 2598–2609. [Google Scholar] [CrossRef] [Green Version]
- Prosolv® ODT. Available online: https://www.jrspharma.com/pharma_en/products-services/excipients/hfe/prosolv-odt-g2.php (accessed on 20 February 2020).
- Parteck® ODT. Available online: http://www.phexcom.cn/uploadfiles/2011126103726995.pdf (accessed on 20 February 2020).
- Fmelt®. Available online: http://www.fujichemical.co.jp/english/newsletter/newsletter_pharma_0802.html (accessed on 20 February 2020).
- SmartEx® QD-50. Available online: http://www.metolose.jp/en/pharmaceutical/smartexr.html (accessed on 20 February 2020).
- Pearlitol® Flash. Available online: https://www.roquette.com/pharma-and-nutraceuticals-coprocessed-mannitol-starch (accessed on 20 February 2020).
- Ludiflash®. Available online: https://pharmaceutical.basf.com/global/en/drug-formulation/products/ludiflash.html (accessed on 20 February 2020).
- Council of Europe. The European Pharmacopoeia, 9th ed.; Council of Europe: Strasburg, France, 2016. [Google Scholar]
- Choudekar, R.L.; Mahajan, M.P.; Sawant, S.D. Validated RP-HPLC method for the estimation of rupatadine fumarate in bulk and tablet dosage form. Pharma Chem. 2012, 4, 1047–1053. [Google Scholar]
- Redasani, V.K.; Kothawade, A.R.; Surana, S.J. Stability indicating RP-HPLC method for simultaneous estimation of rupatadine fumarate and montelukast sodium in bulk and tablet dosage form. J. Anal. Chem. 2014, 69, 384–389. [Google Scholar] [CrossRef]
- Rele, R.V.; Mali, R.N. New validated RP-HPLC method for quantification of rupatadine fumarate impurities in solid dosage form supported by forced degradation studies. Der Pharm. Lett. 2016, 8, 66–72. [Google Scholar]
- Stoltenberg, I.; Breitkreutz, J. Orally disintegrating mini-tablets (ODMTs)—A novel solid oral dosage form for paediatric use. Eur. J. Pharm. Biopharm. 2011, 78, 462–469. [Google Scholar] [CrossRef]
- FDA; CDER. Guidance for Industry—Orally Disintegrating Tablets. 2008. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm070578.pdf (accessed on 20 January 2020).
- Amelian, A.; Szekalska, M.; Ciosek, P.; Basa, A.; Winnicka, K. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray dryling. Acta Pharm. 2017, 67, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łabańska, M.; Ciosek-Skibińska, P.; Wróblewski, W. Critical evaluation of laboratory potentiometric electronic tongues for pharmaceutical analysis—An overview. Sensors 2019, 19, 5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamizadeh, S.; Brockow, K.; Ring, J. Rupatadine: Efficacy and safety of a non-sedating antihistamine with PAF-antagonist effects. Allergo J. Int. 2014, 23, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Mullol, J.; Gonzalez-Nunez, V.; Bachert, C. Rupatadine: Global safety evaluation in allergic rhinitis and urticaria. Expert Opin. Drug Saf. 2016, 15, 1439–1448. [Google Scholar]
- Rao Sudhakara, M.; Dwarakanatha Reddy, D.; Murthy, P.S.N. Rupatadine: Pharmacological profile and its use in the treatment of allergic rhinitis. Indian J. Otolaryngol. Head Neck Surg. 2009, 61, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Picado, C.S. Rupatadine: Pharmacological profile and its use in the treatment of allergic disorders. Expert Opin. Pharmacoter. 2006, 7, 1989–2001. [Google Scholar] [CrossRef]
- Merlos, M.; Giral, M.; Balsa, D.; Ferrando, R.; Queralt, M.; Puigdemont, A.; García-Rafanell, J.; Forn, J. Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF). J. Pharmacol. Exp. Ther. 1997, 280, 114–121. [Google Scholar]
- Chuch, M.K. Efficacy and tolerability of rupatadine at four times the recommended dose against histamine- and platelet-activating factor-induced flare responses and ex vivo platelet aggregation in healthy males. Br. J. Dermatol. 2010, 163, 1330–1332. [Google Scholar]
- Izquierdo, I.; Valero, A.; García, O.; Pérez, I.; Mullol, J.; Van Cauwenberge, P. Clinical efficacy of rupatadine in allergic rhinitis under ARIA criteria: Pooled analysis. Allergy Clin. Immunol. Int. 2005, 1, 271–277. [Google Scholar]
- Rupafin®. Available online: https://www.drugs.com/uk/rupafin-10mg-tablets-leaflet.html (accessed on 20 February 2020).
- FDA Inactive Ingredients Database. Available online: https://search.fda.gov/search?utf8=%E2%9C%93&affiliate=fda1&query=ethylcellulose&commit=Search (accessed on 20 February 2020).
- WHO. Available online: https://apps.who.int/iris/bitstream/handle/10665/42601/WHO_TRS_913.pdf (accessed on 20 February 2020).
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009; pp. 262–267. [Google Scholar]
- Ethylcellulose. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24832091#section=ProbableRoutes-of-Human-Exposure (accessed on 20 April 2020).
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A pharmaceutical excipient with multidirectional application in drug dosage forms development. Materials 2019, 12, E3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safety & Toxicity of Excipients for Paediatrics, STEP Database. Available online: http://www.eupfi.org/step-database-info/ (accessed on 26 May 2020).
- Canadian List of Acceptable Non-Medicinal Ingredients. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/applications-submissions/product-licensing/compendium-monographs.html. (accessed on 26 May 2020).
- Surelease®. Available online: https://www.colorcon.com/products-formulation/all-products/filmcoatings/sustained-release/surelease (accessed on 20 February 2020).
- Aquacoat® ECD. Available online: http://www.fmcbiopolymer.com/Pharmaceutical/Products/Aquacoat.aspx (accessed on 20 February 2020).
- Müller, K.; Fingueroa, C.; Martinez, C.; Madel, M.; Obreque, E.; Peña-Neira, A.; Morales-Bozo, I.; Toledo, H.; Lopez-Solis, R.O. Measurement of saliva volume in the mouth of members of a trained sensory panel using a beetroot (Beta vulgaris) extract. Food Qual. Prefer. 2010, 21, 569–574. [Google Scholar] [CrossRef]
- Ali, J.; Zgair, A.; Hameed, G.D.S.; Garnet, M.C.; Roberts, C.J.; Vurley, J.C.; Gershkovich, P. Application of biorelevant saliva-based dissolution for optimization of orally disintegrating formulations of felodipin. Int. J. Pharm. 2019, 30, 228–236. [Google Scholar] [CrossRef]
- Brniak, W.; Jachowicz, R.; Pelka, P. The practical approach to the evaluation of methods used to determinate the disintegration time of orally disintegrating tablets (ODTs). Saudi Pharm. J. 2015, 23, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Rupatadine Fumarate. Available online: https://www.drugbank.ca/salts/DBSALT001922 (accessed on 20 February 2020).
- Henríquez, L.C.; Redondo, G.M.; Zúñiga, R.V.; Berrocal, G.C. Identification of rupatadine fumarate polymorphic crystalline forms in pharmaceutical raw materials. AJST 2018, 9, 7482–7487. [Google Scholar]
- Henríquez, L.C.; Zúñiga, R.V.; Redondo, G.M.; Berrocal, G.C.; Vargas, G.H. Determination of the impact caused by direct compression on the crystalline state of rupatadine fumarate 10 mg tablets. Int. J. Pharm. Technol. Biotechnol. 2019, 6, 1–12. [Google Scholar]
- Mohamed-Ahmed, A.H.; Soto, J.; Ernest, T.; Tuleu, C. Non-human tools for the evaluation of bitter taste in the design and development of medicines: A systematic review. Drug Discov. Today 2016, 21, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.; Kirsanov, D.; Legin, A.; Rudnitskaya, A.; Saunders, K. Assessing taste without using humans: Rat brief access aversion model and electronic tongue. Int. J. Pharm. 2012, 435, 137–139. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Clapham, D.; Seleznev, B.; Lord, K.; Vlasov, Y. Electronic tongue for pharmaceutical analytics: Quantification of tastes and masking effects. Anal. Bioanal. Chem. 2004, 380, 36–45. [Google Scholar] [CrossRef]
- Wesoły, M.; Cal, K.; Ciosek, P.; Wróblewski, W. Influence of dissolution-modifying excipients in various pharmaceutical formulations on electronic tongue results. Talanta 2017, 162, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wesoły, M.; Zabadaj, M.; Amelian, A.; Winnicka, K.; Wróblewski, W.; Ciosek, P. Tasting cetirizine-based microspheres with an electronic tongue. Sens. Actuators B 2017, 238, 1190–1198. [Google Scholar] [CrossRef]
- Krishnan, A.; Williams, L.J.; McIntosh, A.R.; Abdi, H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage 2011, 56, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Wesoły, M.; Ciosek-Skibińska, P. Comparison of performance of various data analysis techniques applied for the classification of pharmaceutical samples by electronic tongue. Sens. Actuators B 2018, 267, 570–580. [Google Scholar] [CrossRef]
- Zabadaj, M.; Szuplewska, A.; Kalinowska, D.; Chudy, M.; Ciosek-Skibińska, P. Studying pharmacodynamic effects in cell cultures by chemical fingerprinting—SIA electronic tongue versus 2D fluorescence soft sensor. Sens. Actuators B 2018, 272, 264–273. [Google Scholar] [CrossRef]
- Zabadaj, M.; Ufnalska, I.; Chreptowicz, K.; Mierzejewska, J.; Wróblewski, W.; Ciosek-Skibińska, P. Performance of hybrid electronic tongue and HPLC coupled with chemometric analysis for the monitoring of yeast biotransformations. Chemometr. Intell. Lab. 2017, 167, 69–77. [Google Scholar] [CrossRef]










| Ingredient [%] | Formulation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | |
| RUP | - | 3.5 | - | 3.5 | - | 3.5 | - | 3.5 | ||||||||
| SUR MP RUP[corresponding to 0.5 mg RUP per one tablet] | - | 7.15 | - | - | 7.15 | - | - | 7.15 | - | - | 7.15 | |||||
| AQ MP RUP[corresponding to 0.5 mg RUP] | - | 8.65 | - | - | 8.65 | - | - | 8.65 | - | - | 8.65 | |||||
| Parteck® ODT | 99 | 95.5 | 91.85 | 90.35 | - | - | - | - | - | - | ||||||
| SmartEx® QD-50 | - | 99 | 95.5 | 91.85 | 90.35 | - | - | - | - | |||||||
| F-Melt C | - | - | - | 99 | 95.5 | 91.85 | 90.35 | - | - | |||||||
| Pearlitol® Flash | - | - | - | - | 99 | 95.5 | 91.85 | 90.35 | ||||||||
| Magnesium stearate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Electrode Number no. | Electrode Type | Ionophore (%, w/w) | Lipophilic Salt (%, w/w) | Plasticizer (%, w/w) | Polymer (%, w/w) |
|---|---|---|---|---|---|
| 1–2 | CSF-D | − | KTFPB (1%) | DOS (66%) | PVC (33%) |
| 3–4 | CSF-N | − | KTFPB (1%) | o-NPOE (66%) | PVC (33%) |
| 5–6 | CSC-D | − | KTpCPB (3%) | DOS (64%) | PVC (33%) |
| 7–8 | CSC-N | − | KTpCPB (3%) | o-NPOE (64%) | PVC (33%) |
| 9–10 | AM-D | Amine ionophore I (5%) | − | DOS (68%) | PVC (27%) |
| 11–12 | MET-N | METRIAN (4%) | − | o-NPOE (66%) | PVC (30%) |
| 13–14 | PC-N | − | DDPC (3%) | o-NPOE (64%) | PVC (33%) |
| 15–16 | AN-N | − | TDMAC (4%) | o-NPOE (66%) | PVC (30%) |
| Powder Mixture | Density [g/mL] | Flow Properties | ||
|---|---|---|---|---|
| Bulk | Tapped | Hausner’s Ratio | Carr’s Index [%] | |
| F1 | 0.58 | 0.72 | 20.45 | 1.26 |
| F2 | 0.58 | 0.71 | 20.45 | 1.26 |
| F3 | 0.56 | 0.70 | 20.40 | 1.27 |
| F4 | 0.57 | 0.68 | 20.20 | 1.24 |
| F5 | 0.51 | 0.65 | 15.38 | 1.28 |
| F6 | 0.52 | 0.65 | 15.38 | 1.28 |
| F7 | 0.50 | 0.63 | 15.39 | 1.29 |
| F8 | 0.49 | 0.62 | 15.25 | 1.30 |
| F9 | 0.56 | 0.67 | 13.25 | 1.16 |
| F10 | 0.56 | 0.66 | 13.24 | 1.16 |
| F11 | 0.54 | 0.62 | 13.23 | 1.17 |
| F12 | 0.52 | 0.61 | 13.22 | 1.15 |
| F13 | 0.48 | 0.57 | 13.10 | 1.15 |
| F14 | 0.48 | 0.58 | 13.10 | 1.15 |
| F15 | 0.45 | 0.54 | 13.05 | 1.19 |
| F16 | 0.44 | 0.53 | 13.13 | 1.16 |
| Parameter | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formulation | ||||||||||||||||
| Weight [mg] * | 13.4 ± 0.2 | 13.7 ± 0.3 | 14.0 ± 0.7 | 13.6 ± 0.5 | 14.1 ± 0.3 | 12.6 ± 0.9 | 12.9 ± 0.6 | 12.5 ± 0.7 | 13.8 ± 0.3 | 13.4 ± 0.2 | 13.5 ± 0.4 | 13.4 ± 0.5 | 14.1 ± 0.2 | 13.4 ± 0.5 | 13.9 ± 0.3 | 13.70 ± 0.4 |
| Thickness * [mm] | 2.01 ± 0.1 | 2.01 ± 0.1 | 1.96 ± 0.2 | 1.94 ± 0.3 | 1.94 ± 0.1 | 1.96 ± 0.2 | 1.82 ± 0.2 | 1.80 ± 0.3 | 2.0 ± 0.1 | 1.98 ± 0.1 | 1.97 ± 0.3 | 1.95 ± 0.3 | 1.97 ± 0.1 | 1.95 ± 0.1 | 1.95 ± 0.3 | 1.93 ± 0.4 |
| Hardness [N] **(by hardness tester) | 15.4 ± 3.4 | 14.4 ± 2.4 | 8.40 ± 4.5 | 8.20 ± 4.7 | 16.8 ± 2.2 | 16.2 ± 2.5 | 8.1 ± 2.1 | 7.8 ± 2.3 | 15.9 ± 2.5 | 15.1 ± 2.1 | 8.1 ± 2.7 | 7.8 ± 2.9 | 16.1 ± 1.2 | 15.8 ± 2.1 | 8.1 ± 3.7 | 7.5 ± 4.2 |
| Hardness [N] **(by texture analyzer) | 15.1 ± 3.9 | 14.6 ± 2.3 | 8.1 ± 4.0 | 8.0 ± 4.4 | 16.7 ± 2.2 | 16.5 ± 2.4 | 8.1 ± 1.5 | 7.8 ± 2.5 | 16.0 ± 2.2 | 15.0 ± 2.0 | 8.2 ± 2.5 | 8.0 ± 2.8 | 16.1 ± 1.1 | 15.9 ± 2.3 | 8.0 ± 3.5 | 7.6 ± 4.1 |
| Friability [%] | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.45 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 |
| Drug content *** [mg] | - | 0.47 ± 0.1 | 0.48 ± 0.3 | 0.43 ± 0.4 | - | 0.45 ± 0.1 | 0.43 ± 0.2 | 0.42 ± 0.3 | - | 0.47 ± 0.1 | 0.45 ± 0.3 | 0.41 ± 0.4 | - | 0.49 ± 0.1 | 0.5 ± 0.1 | 0.40 ± 0.2 |
| % of declared dose | - | 94 | 96 | 86 | - | 90 | 86 | 84 | - | 94 | 90 | 82 | - | 98 | 100 | 0.80 |
| Volunteer | Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2 | F3 | F4 | F6 | F7 | F8 | F10 | F11 | F12 | F14 | F15 | F16 | |
| A | 3 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 1 |
| B | 3 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 |
| C | 2 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 1 |
| D | 2 | 0 | 1 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| E | 3 | 0 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 3 | 0 | 1 |
| F | 2 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, K.; Ciosek-Skibińska, P.; Lenik, J.; Srčič, S.; Basa, A.; Winnicka, K. Utilization of Ethylcellulose Microparticles with Rupatadine Fumarate in Designing Orodispersible Minitablets with Taste Masking Effect. Materials 2020, 13, 2715. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13122715
Wasilewska K, Ciosek-Skibińska P, Lenik J, Srčič S, Basa A, Winnicka K. Utilization of Ethylcellulose Microparticles with Rupatadine Fumarate in Designing Orodispersible Minitablets with Taste Masking Effect. Materials. 2020; 13(12):2715. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13122715
Chicago/Turabian StyleWasilewska, Katarzyna, Patrycja Ciosek-Skibińska, Joanna Lenik, Stanko Srčič, Anna Basa, and Katarzyna Winnicka. 2020. "Utilization of Ethylcellulose Microparticles with Rupatadine Fumarate in Designing Orodispersible Minitablets with Taste Masking Effect" Materials 13, no. 12: 2715. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13122715