Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Wood Impregnation with Compatibilizer
2.3. Composites Preparation
2.4. Methodology
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Wide-Angle X-ray Diffraction (WAXD)
2.4.3. Solid-State 1H NMR
2.4.4. Thermogravimetry (TGA)
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Mechanical Properties
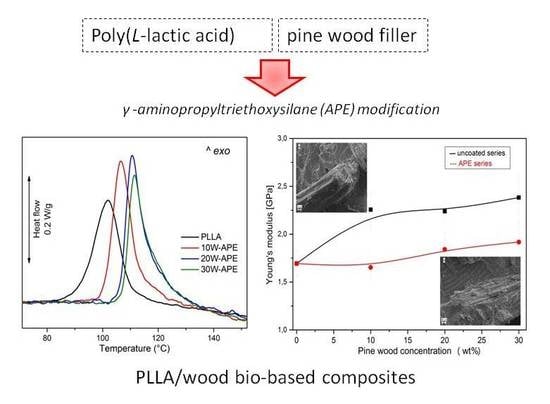
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faludi, G.; Dora, G.; Renner, K.; Móczó, J.; Pukánszky, B. Improving interfacial adhesion in PLLA/wood biocomposites. Compos. Sci. Technol. 2016, 89, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, M.L.; Androsch, R. Thermal Properties of Bio-based Polymers; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Di Lorenzo, M.L.; Androsch, R. Influence of alfa’/alfa-crystal polymorphism on properties of poly(l-lactic acid). Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Synthesis, Structure and Properties of Poly(lactic acid); Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Mysiukiewicz, O.; Barczewski, M. Utilization of linseed cake as a postagricultural functional filler for poly(lactic acid) green composites. J. Appl. Polym. Sci. 2019, 136, 10. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Kloziński, A. Influence of Time and Oil Content within the Filler. Polymers 2019, 11, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barczewski, M.; Mysiukiewicz, O.; Skórczewska, K.; Szulc, J.; Kloziński, A. Poly(lactic acid) green composites filled with linseed cake as an agricultural waste filler. Influence of oil content within the filler on the rheological behavior. J. Appl. Polym. Sci. 2019, 136, 47651. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive Plasticizing Polylactic acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Burzic, I.; Pretschuh, C.; Kaineder, D.; Eder, G.; Smilek, J.; Másilko, J.; Kateryna, W. Impact modification of PLLA using biobased biodegradable PHA biopolymers. Eur. Polym. J. 2019, 114, 32–38. [Google Scholar] [CrossRef]
- Sun, C.; Chang, L.; Tan, H.; Zhang, Y. Enhancing the durability of poly(lactic acid) composites by nucleated modification. Polym. Int. 2019, 68, 1450–1459. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Mechanical and thermal properties of coconut shell powder filled polylactic acid biocomposites: Effects of the filler content and silane coupling agent. J. Polym. Res. 2012, 19, 9859. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical Properties of Biodegradable Composites from Poly Lactic Acid (PLA) and Microcrystalline Cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Awal, A.; Rana, M.; Sain, M. Thermorheological and mechanical properties of cellulose reinforced PLLA bio-composites. Mech. Mater. 2015, 80, 87–95. [Google Scholar] [CrossRef]
- Clemons, C.M.; Caulfield, D.F. Wood flour. In Functional Fillers for Plastics; Wiley-VCH: Weinheim, Germany, 2005; pp. 249–270. [Google Scholar]
- Barczewski, M.; Matykiewicz, D.; Krygier, A.; Andrzejewski, J.; Skórczewska, K. Characterization of poly(lactic acid) biocomposites filled with chestnut shell waste. J. Mater. Cycles Waste Manag. 2018, 20, 914–924. [Google Scholar] [CrossRef]
- Csizmadia, R.; Faludi, G.; Renner, K.; Móczó, J.; Pukánszky, B. PLA/wood biocomposites: Improving composite strength by chemical treatment of the fibers. Compos. Part. A Appl. Sci. Manuf. 2013, 53, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wei, J.; Xu, S.; Zhu, Q.; Liu, W.; Qiu, Y.; Jiang, Q. Full-degradable composites reinforced by the low temperature treated cotton fabrics with enhanced strength and interfacial bonding. Compos. B Eng. 2019, 177, 107269. [Google Scholar] [CrossRef]
- Shih, Y.F.; Lai, Z.Z. Green Composites Based on Poly (Lactic Acid) and Bamboo Fiber: Flame Retardancy, Thermal, and Mechanical Properties. Springer Proc. Phys. 2020, 242, 61–69. [Google Scholar]
- Muthuraj, R.; Lacoste, C.; Lacroix, P.; Bergeret, A. Sustainable thermal insulation biocomposites from rice husk, wheat husk, wood fibers and textile waste fibers: Elaboration and performances evaluation. Ind. Crops. Prod. 2019, 135, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumari, S.; Rai, B.; Das, R.; Kumar, G. Effect of nano-cellulosic fiber on mechanical and barrier properties of polylactic acid (PLA) green nanocomposite film. Mater. Res. Express 2019, 6, 125108. [Google Scholar] [CrossRef]
- Wu, H.; Hao, M. Strengthening and toughening of polylactide/sisal fiber biocomposites via in-situ reaction with epoxy-functionalized oligomer and poly (butylene-adipate-terephthalate). Polymers 2019, 11, 1747. [Google Scholar] [CrossRef] [Green Version]
- Błędzki, A.K.; Jaszkiewicz, A.; Scherzer, D. Mechanical properties of PLA composites with man-made cellulose and abaca fibres. Compos. Part. A Appl. Sci. Manuf. 2009, 40, 404–412. [Google Scholar] [CrossRef]
- Stevanovic, T. Chemical Composition and Properties of Wood. In Lignocellulosic Fibers and Wood Handbook; Scrivener Publishing LLC: Beverly, MA, USA, 2016; pp. 49–106. ISBN 9781118773727. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications, 2nd ed.; Elsevier: San Diego, CA, USA, 1993. [Google Scholar]
- Dányádi, L.; Renner, K.; Móczó, J.; Pukánszky, B. Wood flour filled polypropylene composites: Interfacial adhesion and micromechanical deformations. Polym. Eng. Sci. 2007, 47, 1246–1255. [Google Scholar] [CrossRef]
- Renner, K.; Kenyó, C.; Móczó, J.; Pukánszky, B. Micromechanical deformation processes in PP/wood composites: Particle characteristics, adhesion, mechanisms. Compos. Part. A Appl. Sci. Manuf. 2010, 41, 1653–1661. [Google Scholar] [CrossRef]
- Pilla, S.; Gong, S.; O’Neill, E.; Rowell, R.M.; Krzysik, A.M. Polylactide-Pine Wood Flour Composites. Polym. Eng. Sci. 2008, 48, 578–587. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M.; Donescu, D. Cellulose Fiber-Reinforced Polylactic Acid. Polym. Compos. 2011, 32, 976–985. [Google Scholar] [CrossRef]
- Gregorova, A.; Hrabalova, M.; Wimmer, R.; Saake, B.; Altaner, C. Poly(lactide acid) composites reinforced with fibers obtained from different tissue types of Picea sitchensis. J. Appl. Polym. Sci. 2009, 114, 2616–2623. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A.; Gandini, A. Modification of cellulose fibers with functionalized silanes: Effect of the fiber treatment on the mechanical performances of cellulose–thermoset composites. J. Appl. Polym. Sci. 2005, 98, 974–984. [Google Scholar] [CrossRef]
- Frone, A.A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar]
- Zhu, J.; Xue, L.; Wei, W.; Mu, C.; Jiang, M.; Zhou, Z. Modification of lignin with silane coupling agent to improve the interface of poly (L-lactic) acid/lignin composites. BioResources 2015, 10, 4315–4325. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zong, X.; Wang, N.; Yan, N.; Shan, X.; Li, J. Preparation of γ-Divinyl-3-Aminopropyltriethoxysilane modified lignin and its application in flame retardant poly(lactic acid). Materials 2018, 11, 1505. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic Transition in Disordered Poly(L-lactide) Crystals Induced by Annealing at Elevated Temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Starkweather, H.W., Jr.; Avakian, P. Internal Motions in Polylactide and Related Polymers. Macromolecules 1993, 26, 5084–5087. [Google Scholar]
- Nozirova, F.; Nazirovb, A.; Jurga, S.; Fua, R. Molecular dynamics of poly(L-lactide) biopolymer studied by wide-line solid-state 1H and 2H NMR spectroscopy. Solid State Nucl. Magn. Reson. 2006, 29, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Thakur, M.; Kean, R.T.; Zupfer, J.M.; Buehler, N.U. Solid State 13C CP-MAS NMR Studies of the Crystallinity and Morphology of Poly(L-lactide). Macromolecules 1996, 29, 8844–8851. [Google Scholar]
- Laredo, E.; Grimau, M.; Bello, A.; Wu, D. Molecular dynamics and crystallization precursors in polylactide and poly(lactide)/CNT biocomposites in the insulating state. Eur. Polym. J. 2013, 49, 4008–4019. [Google Scholar] [CrossRef]
- Kovalakova, M.; Olcak, D.; Hronsky, V.; Vrabel, P.; Fricova, O.; Chodak, I.; Alexy, P.; Sucik, G. Morphology and molecular mobility of PLLAsticized polylactic acid studied using solid-state 13C- and 1H-NMR spectroscopy. J. Appl. Polym. Sci. 2016, 133, 43517. [Google Scholar]
- Olčák, D.; Hronský, V.; Kovalaková, M.; Vrábel, P.; Chodák, I.; Alexy, P. High-Resolution Solid-State NMR Characterization of Morphology in Annealed Polylactic Acid. Int. J. Polym. Anal. Ch. 2015, 20, 396–405. [Google Scholar] [CrossRef]
- Suganuma, K.; Horiuchi, K.S.K.; Matsuda, H.; Cheng, H.N.; Aoki, A.; Asakura, T. 1NMR analysis and chemical shift calculations of poly(lactic acid) dimer model compounds with different tacticities. Polym. J. 2012, 44, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Thakur, K.A.M.; Kean, R.T.; Hall, E.S.; Kolstad, J.J.; Munson, E.J. 1H NMR Spectroscopy in the Analysis and Characterization of Poly(lactide). Int. J. Polym. Anal. Ch 1997, 4, 379–391. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, L.; Ma, S.; Yang, Y.; Zhang, C.; Tang, Z.; Zhu, J. Effect of castor oil enrichment layer produced by reaction on the properties of PLLA/HDI-g-starch blends. Carbohydr. Polym. 2013, 94, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A Renewable Lignin−Lactide Copolymer and Application in Biobased Composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Hayakawa, Y.; Nishida, M. Solid-State Nuclear Magnetic Resonance (NMR) and Nuclear Magnetic Relaxation Time Analyses of Molecular Mobility and Compatibility of Plasticized Polyhydroxyalkanoates (PHA) Copolymers. Polymers 2018, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Jurga, K.; Fojud, Z.; Woźniak-Braszak, A. NMR strong off-resonance irradiation without sample overheating. Solid State. Nucl. Magn. Reson. 2004, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.S.; Powers, J. Topics in Polymer Physics; Imperial College Press: London, UK, 2006. [Google Scholar]
- Gordobil, O.; Egüés, I.; Labidi, J. Modification of Eucalyptus and Spruce organosolv lignins with fatty acids to use as filler in PLLA. React. Funct. Polym. 2016, 104, 45–52. [Google Scholar] [CrossRef]
- Angelini, S.; Cerruti, P.; Immirzi, B.; Santagata, G.; Scarinzi, G.; Malinconico, M. From biowaste to bioresource: Effect of a lignocellulosic filler on the properties of poly(3-hydroxybutyrate). Int. J. Biol. Macromol. 2014, 71, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenerg. 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Luhr, C.; Pecenka, R. Development of a model for the fast analysis of polymer mixtures based on cellulose, hemicellulose (xylan), lignin using thermogravimetric analysis and application of the model to poplar wood. Fuel 2020, 277, 118169. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). Eur Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Hermans, P.H.; Weidinger, A. On the determination of the crystalline fraction of polyethylenes from X-ray diffraction. Makromol. Chem. 1961, 44, 24–36. [Google Scholar] [CrossRef]
- Baranowski, M.; Woźniak-Braszak, A.; Jurga, K. High homogeneity B(1) 30.2 MHz Nuclear Magnetic Resonance Probe for Off-Resonance Relaxation Times Measurements. J. Magn. Reson. 2011, 208, 163–166. [Google Scholar] [CrossRef]
- Czechowski, T.; Baranowski, M.; Woźniak-Braszak, A.; Jurga, K.; Jurga, J.; Kędzia, P. The instrument set for generating fast adiabatic passage. Appl. Magn. Reson. 2012, 43, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679. [Google Scholar] [CrossRef]
- Abragam, A. The Principles of Nuclear Magnetism; Oxford University Press: Oxford, UK, 1961. [Google Scholar]
- Slichter, C. Principles of Magnetic Resonance; Springer Series in Solid-State Sciences; Springer: Heideberg, Germany, 1978. [Google Scholar]
- Makrocka-Rydzyk, M.; Woźniak-Braszak, A.; Jurga, K.; Jurga, S. Local motions in poly(ethylene-co-norbornene) studied by 1H NMR Relaxometry. Solid State Nucl. Magn. Reson. 2015, 71, 67–72. [Google Scholar]
- Beckmann, P.A. Spectral densities and nuclear spin relaxation in solids. Phys. Rep. 1988, 171, 85–128. [Google Scholar] [CrossRef] [Green Version]
- Barczewski, M.; Chmielewska, D.; Dobrzyńska-Mizera, M.; Dudziec, B.; Sterzyński, T. Thermal stability and flammability of polypropylene-silsesquioxane nanocomposites. Int. J. Polym. Anal. Charact. 2014, 19, 500–509. [Google Scholar] [CrossRef]
- Organisation Internationale de Normalisation. Plastics—Determination of Tensile Properties—Part 1: General Principles; Organisation Internationale de Normalisation: Geneva, Switzerland, 2012. [Google Scholar]
- Organisation Internationale de Normalization. Plastics—Determination of Tensile-Impact Strength; Organisation Internationale de Normalisation: Geneva, Switzerland, 2004. [Google Scholar]
- Wu, W.; Wu, G.; Zhang, H. Effect of wood flour as nucleating agent on the isothermal crystallization of poly(lactic acid): Material Behavior. Polym. Advan. Technol. 2016, 28, 2. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Isotactic polypropylene modified with sorbitol-based derivative and siloxane-silsesquioxane resin. J. Appl. Polym. Sci. 2016, 85, 62–71. [Google Scholar] [CrossRef]
- Delpouve, N.; Delbreilh, L.; Stoclet, G.; Saiter, A.; Dargent, E. Structural Dependence of the Molecular Mobility in the Amorphous Fractions of Polylactide. Macromolecules 2014, 47, 5186–5197. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewski, J.; Skórczewska, K.; Kloziński, A. Improving the Toughness and Thermal Resistance of Polyoxymethylene/Poly(lactic acid) Blends: Evaluation of Structure–Properties Correlation for Reactive Processing. Polymers 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′- and α-crystals of poly(l-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar]
- Chen, W.; Reichert, D.; Miyoshi, T. Helical Jump Motions of Poly(l-Lactic Acid) Chains in the α Phase as Revealed by Solid-State NMR. Phys. Chem. B 2015, 119, 4552–4563. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F. Solid–state 13C NMR analyses of the structures of crystallized and quenched poly(lactide)s: Effects of crystallinity, water absorption, hydrolytic degradation, and tacticity. Polymer 2010, 51, 2215–2220. [Google Scholar] [CrossRef]
- Woźniak-Braszak, A.; Knitter, M.; Markiewicz, E.; Ingram, W.F.; Spontak, R.J. Effect of Composition on the Molecular Dynamics of Biodegradable Isotactic Polypropylene/Thermoplastic Starch Blends. ACS Sustain. Chem. Eng. 2019, 7, 16050–16059. [Google Scholar] [CrossRef]
- Makrocka-Rydzyk, M.; Wypych, A.; Dobies, M.; Jancelewicz, M.; Jurga, S.; Cho, H.Y.; Gao, H.; Matyjaszewski, K. Molecular dynamics in PBA/PEO miktoarm star copolymers. Polymer 2013, 54, 3341–3349. [Google Scholar] [CrossRef]
- Orozbaev, B.; Fojud, Z.; Makrocka-Rydzyk, M.; Schroeder, G.; Jurga, S. Molecular Dynamics of Podand Studied by Broadband Dielectric and Nuclear Magnetic Resonance Spectroscopies. Macromol. Chem. Phys. 2007, 208, 2121–2127. [Google Scholar] [CrossRef]
- Rachocki, A.; Tritt-Goc, J. The Molecular Origin of Nuclear Magnetic Relaxation in Methyl Cellulose and Hydroxypropylmethyl Cellulose. J. Polym. Res. 2006, 13, 201–206. [Google Scholar] [CrossRef]
- Rachocki, A.; Markiewicz, E.; Tritt-Goc, J. Dielectric Relaxation in Cellulose and its Derivatives. Acta Phys. Pol. A 2005, 108, 137–146. [Google Scholar] [CrossRef]
- Tang, H.R.; Belton, P.S. Molecular Dynamics of Polycrystalline Cellobiose Studied by Solid-State NMR. Solid State Nucl Magn Reson 2002, 21, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Einfeldt, J.; Kwasniewski, A. Characterization of different types of cellulose by dielectric spectroscopy. Cellulose 2002, 9, 225–238. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. Int. J. Spectrosc. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic Oxidation of Lignin in Solvent Systems for Production of Renewable Chemicals: A Review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, S.; Zhao, Y.; Huang, S.; Zhao, J. Molecular docking and molecular dynamics simulation analyses of urea with ammoniated and ammoxidized lignin. J. Mol. Graph. Model 2017, 71, 58–69. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of Lignin Polymer materials by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petridis, L.; Schulz, R.; Smith, J.C. Simulation Analysis of the Temperature Dependence of Lignin Structure and Dynamics. J. Am. Chem. Soc. 2011, 133, 20277–20287. [Google Scholar] [CrossRef] [PubMed]
- Ahvazi, B.; Argyropoulos, D.S. Proton spin-lattice relaxation time measurements of solid wood and its constituents as a function of pH: Part I. Wood Sci. Technol. 2000, 34, 45–53. [Google Scholar] [CrossRef]
- Varol, N.; Monnier, X.; Delbreilh, L.; Saiter, A.; Fatyeyeva, K.; Dargent, E. Highlight of primary and secondary relaxations in amorphous stereocomplex polylactides. Express Polym. Lett. 2020, 14, 48–62. [Google Scholar] [CrossRef]
- Henry, F.; Costa, L.C.; Devassine, M. The evolution of poly(lactic acid) degradability by dielectric spectroscopy measurements. Eur. Polym. J. 2005, 41, 2122–2126. [Google Scholar]
- Nishida, M.; Tanaka, T.; Tanaka, T.; Hayakawa, Y. Nucleating and plasticization effects in drawn poly(lactic acid) fiber during accelerated weathering degradation. Polymers 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Glova, A.D.; Falkovich, S.G.; Larin, S.V.; Mezhenskaia, D.A.; Lukasheva, N.V.; Nazarychev, V.M.; Tolmachev, D.A.; Mercurieva, A.A.; Kenny, J.M.; Lyulin, S.V. Poly(lactic acid)-based nanocomposites filled with cellulose nanocrystals with modified surface: All-atom molecular dynamics simulations. Polym. Int. 2016, 65, 892–898. [Google Scholar] [CrossRef]
- Herc, A.S.; Włodarska, M.; Nowacka, M.; Bojda, J.; Szymański, W.; Kowalewska, A. Supramolecular interactions between polylactide and model cyclosiloxanes with hydrogen bonding-capable functional groups. Express Polym. Lett. 2020, 14, 134–153. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface Chemical Functionalization of Cellulose Nanocrystals by 3-Aminopropyltriethoxysilane. Int. J. Bio. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Gupta, M.C.; Deshmukh, V.G. Thermal oxidative degradation of poly(lactic acid), Part II: Molecular weight and electronic spectra during isothermal heating. Coll. Polym. Sci. 1982, 260, 514–517. [Google Scholar] [CrossRef]
- El-Sabbagh, A. Effect of coupling agent on natural fibre in natural fibre/polypropylene composites on mechanical and thermal behaviour. Compos. Part B 2014, 57, 126–135. [Google Scholar] [CrossRef]
- Espinach, F.X.; Boufi, S.; Delgado-Aguilar, M.; Julian, F.; Mutje, P.; Mendez, J.A. Composites from poly(lactic acid) and bleached chemical fibres: Thermal properties. Compos. Part B 2018, 134, 169–176. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, T.H.; Back, J.H.; Jang, S.W.; Kim, H.J.; Skrifvars, M. Phenyl silane treatment and carding process to improve the mechanical, thermal and water-absorption properties of regenerated cellulose lyocell/polylactic acid bio-composites. Compos. Part B 2019, 167, 387–395. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. The effect of morphology and chemical characteristics of cellulose reinforcements on the crystallinity of polylactic acid. J. Appl. Polym. Sci. 2006, 101, 300–310. [Google Scholar] [CrossRef]
- Kristiansen, M.; Werner, M.; Tervoort, T.; Smith, P. The Binary System Isotactic Polypropylene/Bis(3,4-dimethylbenzylidene)sorbitol: Phase Behavior, Nucleation, and Optical Properties. Macromolecules 2003, 36, 5150–5156. [Google Scholar] [CrossRef]
- Balzano, L.; Rastogi, S.; Gerrit, W.; Peters, M. Flow Induced Crystallization in Isotactic Polypropylene−1,3:2,4-Bis(3,4-dimethylbenzylidene)sorbitol Blends: Implications on Morphology of Shear and Phase Separation. Macromolecules 2008, 41, 399–408. [Google Scholar] [CrossRef]
- Hyla, I.; Śleziona, J. Kompozyty. In Elementy Mechaniki i Projektowania; Publishing House of Silesian University of Technology: Gliwice, Poland, 2004. [Google Scholar]
- Demjen, Z.; Pukanszky, B.; Nagy, J. Evaluation of interfacial interaction in polypropylene/surface treated CaCO3 composites. Compos. Part A 1998, 29, 323–329. [Google Scholar] [CrossRef]
- Espino-Perez, E.; Bras, J.; Ducruet, V.; Guinault, A.; Dufresne, A.; Domenek, S. Influence of chemical surface modification of cellulose nanowhiskers on thermal, mechanical, and barrier properties of poly(lactide) based bionanocomposites. Eur. Polym. J. 2013, 49, 3144–3154. [Google Scholar] [CrossRef]









| Designation | Mass Concentration (wt %) | ||
|---|---|---|---|
| PLLA | Wood | Compatibilized Wood | |
| PLLA | 100 | 0 | 0 |
| PLLA/10 W | 90 | 10 | 0 |
| PLLA/20 W | 80 | 20 | 0 |
| PLLA/30 W | 70 | 30 | 0 |
| PLLA/10 W/APE | 90 | 0 | 10 |
| PLLA/20 W/APE | 80 | 0 | 20 |
| PLLA/30 W/APE | 70 | 0 | 30 |
| Sample | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHm (J/g) | Tc (°C) | Xc (%) | mR (%) |
|---|---|---|---|---|---|---|---|
| PLLA | 67 | 98 | 177 | 55 | 102 | 5 | 0 |
| PLLA/10 W | 67 | 93 | 178 | 65 | 110 | 7 | 2 |
| PLLA/20 W | 66 | 90 | 174 | 62 | 111 | 11 | 4 |
| PLLA/30 W | 65 | 89 | 175 | 60 | 111 | 12 | 8 |
| PLLA/10 W/APE | 67 | 94 | 177 | 56 | 107 | 7 | 3 |
| PLLA/20 W/APE | 66 | 90 | 175 | 57 | 111 | 14 | 5 |
| PLLA/30 W/APE | 66 | 89 | 175 | 59 | 112 | 14 | 7 |
| Hindered Rotation of Methyl Groups CH3 Around Their Axes Symmetries | Local Motions of Polymer Chains | ||||||
|---|---|---|---|---|---|---|---|
| Sample | T1L/T1S | τ0 (s) | Ea (kJ/mol) | β | τ0 (s) | Ea (kJ/mol) | β |
| PLLA | T1L | 5.3 × 10−13 | 12.5 | 0.4 | 2.1 × 10−16 | 37.3 | 0.2 |
| PLLA | T1S | 4.8 × 10−12 | 13.9 | 0.4 | 4.9 × 10−16 | 33.3 | 0.2 |
| PLLA/10 W | T1L | 5.4 × 10−12 | 10.7 | 0.2 | N/A | ||
| PLLA/20 W | T1L | 5.9 × 10−12 | 10.0 | 0.2 | N/A | ||
| PLLA/30 W | T1L | 6.0 × 10−12 | 11.1 | 0.2 | N/A | ||
| PLLA/10 W/APE | T1L | 8.4 × 10−13 | 14.1 | 0.2 | 3.5 × 10−16 | 58.2 | 0.1 |
| PLLA/20 W/APE | T1L | N/A | N/A | ||||
| PLLA/30 W/APE | T1L | N/A | N/A | ||||
| Wood * | T1L | 8.2 × 10−11 | 6.7 | 0.3 | 2.2 × 10−12 | 22.4 | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyńska-Mizera, M.; Knitter, M.; Woźniak-Braszak, A.; Baranowski, M.; Sterzyński, T.; Di Lorenzo, M.L. Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites. Materials 2020, 13, 3776. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13173776
Dobrzyńska-Mizera M, Knitter M, Woźniak-Braszak A, Baranowski M, Sterzyński T, Di Lorenzo ML. Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites. Materials. 2020; 13(17):3776. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13173776
Chicago/Turabian StyleDobrzyńska-Mizera, Monika, Monika Knitter, Aneta Woźniak-Braszak, Mikołaj Baranowski, Tomasz Sterzyński, and Maria Laura Di Lorenzo. 2020. "Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites" Materials 13, no. 17: 3776. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13173776