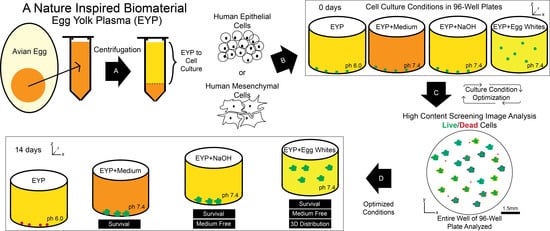
3D Cell Culture of Human Salivary Glands Using Nature-Inspired Functional Biomaterials: The Egg Yolk Plasma and Egg White
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Preparation of Egg Yolk Plasma (EYP) and Egg White (EW) for Cell Culture
2.2. Protein Quantification
2.3. SDS-PAGE and Its Staining with Coomassie and Suddan Black B
2.4. HuSG-Fibro and NS-SV-AC Cell Culture
2.5. Culture of HuSG-Fibro and NS-SV-AC Cells in Biomaterials
2.6. Live/Dead Staining, Imaging and Analysis
2.7. pH Measurements and pH Modification of Egg Yolk Plasma (EYP)
2.8. Statistical Analysis
3. Results
3.1. Isolation and Characteristics of Egg Yolk Plasma (EYP)
3.2. In EYP, NS-SV-AC, or HuSG-Fibro Required at Least 30% Commercial Medium for Cell Survival
3.3. Culture Medium Modified EYP’s pH; so Did NaOH and EW
3.4. In EYP + NaOH (EYP 7.4) NS-SV-AC and HuSG-Fibro Survived without Commercial Medium
3.5. When EYP was Mixed with Egg White (No Commercial Medium or Supplements), Salivary Cells Proliferated and Form Spheroids in 3D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A









References
- Miranda-Rius, J.; Brunet-Llobet, L.; Lahor-Soler, E.; Farré, M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int. J. Med. Sci. 2015, 12, 811–824. [Google Scholar]
- Turner, M.D.; Ship, J.A. Dry Mouth and Its Effects on the Oral Health of Elderly People. J. Am. Dent. Assoc. 2007, 138, S15–S20. [Google Scholar]
- Seo, Y.J.; Lilliu, M.A.; Abu Elghanam, G.; Nguyen, T.T.; Liu, Y.; Lee, J.C.; Presley, J.F.; Zeitouni, A.; El-Hakim, M.; Tran, S.D. Cell culture of differentiated human salivary epithelial cells in a serum-free and scalable suspension system: The salivary functional units model. J. Tissue Eng. Regen. Med. 2019, 13, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mui, B.; Mehrabzadeh, M.; Chea, Y.; Chaudhry, Z.; Chaudhry, K.; Tran, S.D. Regeneration of tissues of the oral complex: Current clinical trends and research advances. J. Can. Dent. Assoc. 2013, 79, d1. [Google Scholar] [PubMed]
- Baum, B.J.; Tran, S.D. Synergy between genetic and tissue engineering: Creating an artificial salivary gland. Periodontol. 2000 2006, 41, 218–223. [Google Scholar] [PubMed]
- Tran, S.D.; Redman, R.S.; Barrett, A.J.; Pavletic, S.Z.; Key, S.; Liu, Y.; Carpenter, A.; Nguyen, H.M.; Sumita, Y.; Baum, B.J.; et al. Microchimerism in salivary glands after blood- and marrow-derived stem cell transplantation. Biol. Blood Marrow Transpl. 2011, 17, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Fang, D.; Liu, Y.; Ramamoorthi, M.; Zeitouni, A.; Chen, W.; Tran, S.D. Three-dimensional organotypic culture of human salivary glands: The slice culture model. Oral Dis. 2016, 22, 639–648. [Google Scholar] [CrossRef]
- Santo, V.E.; Rebelo, S.P.; Estrada, M.F.; Alves, P.M.; Boghaert, E.; Brito, C. Drug screening in 3D in vitro tumor models: Overcoming current pitfalls of efficacy read-outs. Biotechnol. J. 2017, 12, 1600505. [Google Scholar] [CrossRef]
- Wu, T.; Yu, G.Y.; Xiao, J.; Yan, C.; Kurihara, H.; Li, Y.F.; So, K.F.; He, R.R. Fostering efficacy and toxicity evaluation of traditional Chinese medicine and natural products: Chick embryo as a high throughput model bridging in vitro and in vivo studies. Pharm. Res. 2018, 133, 21–34. [Google Scholar] [CrossRef]
- Steffens, D.; Braghirolli, D.I.; Maurmann, N.; Pranke, P. Update on the main use of biomaterials and techniques associated with tissue engineering. Drug Discov. Today 2018, 23, 1474–1488. [Google Scholar] [CrossRef]
- Renth, A.N.; Detamore, M.S. Leveraging “Raw Materials” as Building Blocks and Bioactive Signals in Regenerative Medicine. Tissue Eng. Part B Rev. 2012, 18, 341–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. Tissue engineering: Still facing a long way ahead. J. Control. Release 2018, 279, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.-N.; Tran, S.D.; Abughanam, G.; Laurenti, M.; Zuanazzi, D.; Mezour, M.A.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; Tamimi, F. Biomaterial surface proteomic signature determines interaction with epithelial cells. Acta Biomater. 2017, 54, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, T.; Fowler, E.W.; Liu, S.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D. ACS Biomater. Sci. Eng. 2016, 2, 2217–2230. [Google Scholar] [CrossRef] [Green Version]
- Shubin, A.D.; Felong, T.J.; Schutrum, B.E.; Joe, D.S.L.; Ovitt, C.E.; Benoit, D.S.W. Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017, 50, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Joraku, A.; Sullivan, C.A.; Yoo, J.J.; Atala, A. Tissue Engineering of Functional Salivary Gland Tissue. Laryngoscope 2005, 115, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Maria, O.M.; Liu, Y.; El-Hakim, M.; Zeitouni, A.; Tran, S.D. The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures. J. Tissue Eng. Regen. Med. 2017, 11, 2643–2657. [Google Scholar] [CrossRef]
- Burford-Mason, A.P.; Dardick, I.; Mackay, A. Collagen gel cultures of normal salivary gland: Conditions for continued proliferation and maintenance of major cell phenotypes in vitro. Laryngoscope 1994, 104, 335–340. [Google Scholar] [CrossRef]
- Maria, O.M.; Zeitouni, A.; Gologan, O.; Tran, S.D. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng. Part A 2011, 17, 1229–1238. [Google Scholar] [CrossRef]
- Szlávik, V.; Szabó, B.; Vicsek, T.; Barabás, J.; Bogdán, S.; Gresz, V.; Varga, G.; O’Connell, B.; Vág, J. Differentiation of Primary Human Submandibular Gland Cells Cultured on Basement Membrane Extract. Tissue Eng. Part A 2008, 14, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Joraku, A.; Sullivan, C.A.; Yoo, J.; Atala, A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation 2007, 75, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Burghartz, M.; Lennartz, S.; Schweinlin, M.; Hagen, R.; Kleinsasser, N.; Hackenberg, S.; Steussloff, G.; Scherzad, A.; Radeloff, K.; Ginzkey, C.; et al. Development of Human Salivary Gland-Like Tissue In Vitro. Tissue Eng. Part A 2018, 24, 301–309. [Google Scholar] [CrossRef]
- Lilliu, M.A.; Seo, Y.J.; Isola, M.; Charbonneau, A.M.; Zeitouni, A.; El-Hakim, M.; Tran, S.D. Natural extracellular matrix scaffolds recycled from human salivary digests: A morphometric study. Oral Dis. 2016, 22, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Seuss-baum, I. Nutritional Evaluation of Egg Compounds. In Bioactive Egg Compounds; Huopalahti, R., López-Fandiño, R., Anton, M., Schade, R., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; pp. 117–144. [Google Scholar]
- Laca, A.; Paredes, B.; Rendueles, M.; Díaz, M. Egg yolk plasma: Separation, characteristics and future prospects. LWT Food Sci. Technol. 2015, 62, 7–10. [Google Scholar] [CrossRef]
- Guilmineau, F.; Krause, I.; Kulozik, U. Efficient Analysis of Egg Yolk Proteins and Their Thermal Sensitivity Using Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis under Reducing and Nonreducing Conditions. J. Agric. Food Chem. 2005, 53, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Mann, M. The chicken egg yolk plasma and granule proteomes. Proteomics 2008, 8, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Anton, M. Composition and Structure of Hen Egg Yolk. In Bioactive Egg Compounds; Huopalahti, R., López-Fandiño, R., Anton, M., Schade, R., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; pp. 1–6. [Google Scholar]
- Zambrowicz, A.; Dabrowska, A.; Bobak, L.; Szoltysik, M. Egg yolk proteins and peptides with biological activity. Postepy Hig. I Med. Dosw. (Online) 2014, 68, 1524–1529. [Google Scholar] [CrossRef]
- Stevens, L. Egg proteins: What are their functions? Sci. Prog. 1996, 79, 65–87. [Google Scholar]
- Walker, N.E. Growth and Development of Chick Embryos Nourished by Fractions of Yolk. J. Nutr. 1967, 92, 111–117. [Google Scholar] [CrossRef]
- Grau, C.R.; Klein, N.W.; Lau, T.L. Total Replacement of the Yolk of Chick Embryos. J. Embryol. Exp. Morphol. 1957, 5, 210–214. [Google Scholar]
- Grau, C.R.; Fritz, H.I.; Walker, N.E.; Klein, N.W. Nutrition studies with chick embryos deprived of yolk. J. Exp. Zool. 1962, 150, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Spratt, N.T. Development in vitro of the early chick blastoderm explanted on yolk and albumen extract saline-agar substrata. J. Exp. Zool. 1947, 106, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Spratt, N.T. Development of the early chick blastoderm on synthetic media. J. Exp. Zool. 1948, 107, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Spratt, N.T. Nutritional requirements of the early chick embryo. I. The utilization of carbohydrate substrates. J. Exp. Zool. 1949, 110, 273–298. [Google Scholar] [CrossRef]
- Spratt, N.T. Nutritional requirements of the early chick embryo. II. Differential nutrient requirements for morphogenesis and differentiation of the heart and brain. J. Exp. Zool. 1950, 114, 375–402. [Google Scholar] [CrossRef]
- Spratt, N.T. Metabolism of the early embryo. Ann. N. Y. Acad. Sci. 1952, 55, 40–50. [Google Scholar] [CrossRef]
- Coon, H.G.; Cahn, R.D. Differentiation in vitro: Effects of Sephadex Fractions of Chick Embryo Extract. Science 1966, 153, 1116–1119. [Google Scholar] [CrossRef]
- Spratt, N.T. Nutritional requirements of the early chick embryo. III. The metabolic basis of morphogenesis and differentiation as revealed by the use of inhibitors. Biol. Bull. 1950, 99, 120–135. [Google Scholar]
- Freshney, R.I. Defined Media and Supplements. In Culture of Animal Cells, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 99–114. [Google Scholar]
- Fujii, D.K.; Gospodarowicz, D. Chicken Egg Yolk-Supplemented Medium and the Serum-Free Growth of Normal Mammalian Cells. Vitro 1983, 19, 811–817. [Google Scholar] [CrossRef]
- Murakami, H.; Okazaki, Y.; Yamada, K.; Omura, H. Egg yolk lipoprotein, a new supplement for the growth of mammalian cells in serum-free medium. Cytotechnology 1988, 1, 159–169. [Google Scholar] [CrossRef]
- Goldstein, R.S. Transplantation of Human Embryonic Stem Cells and Derivatives to the Chick Embryo. In Human Embryonic Stem Cell Protocols; Turksen, K., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 367–385. [Google Scholar]
- Goldstein, R.S.; Drukker, M.; Reubinoff, B.E.; Benvenisty, N. Integration and differentiation of human embryonic stem cells transplanted to the chick embryo. Dev. Dyn. 2002, 225, 80–86. [Google Scholar] [CrossRef]
- Auerbach, R. Models for Angiogenesis. In Angiogenesis: An Integrative Approach From Science to Medicine; Figg, W.D., Folkman, J., Eds.; Springer US: Boston, MA, USA, 2008; pp. 299–312. [Google Scholar]
- Kaipparettu, B.A.; Kuiatse, I.; Tak-Yee Chan, B.; Benny Kaipparettu, M.; Lee, A.V.; Oesterreich, S. Novel egg white-based 3-D cell culture system. BioTechniques 2008, 45, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Mousseau, Y.; Mollard, S.; Qiu, H.; Richard, L.; Cazal, R.; Nizou, A.; Vedrenne, N.; Remi, S.; Baaj, Y.; Fourcade, L.; et al. In vitro 3D angiogenesis assay in egg white matrix: Comparison to Matrigel, compatibility to various species, and suitability for drug testing. Lab. Investig. A J. Tech. Methods Pathol. 2014, 94, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodil, A.; Laca, A.; Paredes, B.; Rendueles, M.; Meana, A.; Diaz, M. Gels prepared from egg yolk and its fractions for tissue engineering. Biotechnol. Prog. 2016, 32, 1577–1583. [Google Scholar] [CrossRef]
- Schmidt, G.; Bessman, M.J.; Hickey, M.D.; Thannhauser, S.J. The concentrations of some constituents of egg yolk in its soluble phase. J. Biol. Chem. 1956, 223, 1027–1031. [Google Scholar]
- Mahadevan, S.; Satyanarayana, T.; Kumar, S.A. Physicochemical studies on the gelation of hen egg yolk; separation of gelling protein components from yolk plasma. J. Agric. Food Chem. 1969, 17, 767–771. [Google Scholar] [CrossRef]
- Gallagher, S.R. One-Dimensional SDS Gel Electrophoresis of Proteins. Curr. Protoc. Mol. Biol. 2012, 97, 10.2A.1–10.2A.44. [Google Scholar] [CrossRef]
- Prat, J.P.; Lamy, J.N.; Weill, J.D. Coloration des lipoprotéines après électrophorèse en gel de polyacrylamide. Bull. Soc. Chim. Biol. 1969, 51, 1367. [Google Scholar]
- Takashima, A. Establishment of Fibroblast Cultures. Curr. Protoc. Cell Biol. 1998, 2.1.1–2.1.12. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Tamatani, T.; Kasai, Y.; Sato, M. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori- mutant deoxyribonucleic acid. Lab. Investig. A J. Tech. Methods Pathol. 1993, 69, 24–42. [Google Scholar]
- Azuma, M.; Sato, M. Morphogenesis of normal human salivary gland cells in vitro. Histol. Histopathol. 1994, 9, 781–790. [Google Scholar]
- Saari, A.; Powrie, W.D.; Fennema, O. Isolation and Characterization of Low-Density Lipoproteins in Native Egg Yolk Plasma. J. Food Sci. 1964, 29, 307–315. [Google Scholar] [CrossRef]
- Mc Bee, L.E.; Cotterill, O.J. Ion-Exchange Chromatography and Electrophoresis of Egg Yolk Proteins. J. Food Sci. 1979, 44, 656–667. [Google Scholar]
- Mann, K. Proteomic analysis of the chicken egg vitelline membrane. Proteomics 2008, 8, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S. Chicken embryo utilization of egg micronutrients. Rev. Bras. Ciênc. Avíc. 2007, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yadgary, L.; Cahaner, A.; Kedar, O.; Uni, Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010, 89, 2441–2452. [Google Scholar] [CrossRef]
- Uni, Z.; Yadgary, L.; Yair, R. Nutritional limitations during poultry embryonic development1. J. Appl. Poult. Res. 2012, 21, 175–184. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Mann, K.; Bourin, M.; Brionne, A.; Nys, Y. Effect of Embryonic Development on the Chicken Egg Yolk Plasma Proteome after 12 Days of Incubation. J. Agric. Food Chem. 2014, 62, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Loe, F.; Blocki, A.; Peng, Y.; Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Deliv. Rev. 2011, 63, 277–290. [Google Scholar] [CrossRef]
- Benny, P.; Badowski, C.; Lane, E.B.; Raghunath, M. Making more matrix: Enhancing the deposition of dermal-epidermal junction components in vitro and accelerating organotypic skin culture development, using macromolecular crowding. Tissue Eng. Part A 2015, 21, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbonneau, A.M.; Al-Samadi, A.; Salo, T.; Tran, S.D. 3D Culture Histology Cryosectioned Well Insert Technology Preserves the Structural Relationship between Cells and Biomaterials for Time-Lapse Analysis of 3D Cultures. Biotechnol. J. 2019, 14, 1900105. [Google Scholar] [CrossRef] [PubMed]
- Shafi, R.; Cotterill, O.J.; Nichols, M.L. Microbial Flora of Commercially Pasteurized Egg Products. Poult. Sci. 1970, 49, 578–585. [Google Scholar] [CrossRef]
- Cunningham, F.E. Egg-Product Pasteurization. In Egg Science and Technology, 4th ed.; Cotterill, O.J., Stadelman, W.J., Eds.; Haworth Press: New York, NY, USA, 1994; pp. 289–321. [Google Scholar]
- Le Denmat, M.; Anton, M.; Gandemer, G. Protein Denaturation and Emulsifying Properties of Plasma and Granules of Egg Yolk as Related to Heat Treatment. J. Food Sci. 1999, 64, 194–197. [Google Scholar] [CrossRef]
- Charbonneau, A.M.; Kinsella, J.M.; Tran, S.D. 3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma. Materials 2019, 12, 3480. [Google Scholar] [CrossRef] [Green Version]
- Patten, B.M. The Gametes and Fertilization. In Early Embryology of the Chick, 5th ed.; Robinson, J., Wagner, J., Eds.; McGraw-Hill: New York, NY, USA, 1971; pp. 11–39. [Google Scholar]
- Bruzual, J.J.; Peak, S.D.; Brake, J.; Peebles, E.D. Effects of Relative Humidity During Incubation on Hatchability and Body Weight of Broiler Chicks from Young Breeder Flocks1. Poult. Sci. 2000, 79, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Noiva, R.M.; Menezes, A.M.C.; Peleteiro, M.C. Influence of temperature and humidity manipulation on chicken embryonic development. BMC Vet. Res. 2014, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Schindler, E.I.; Brown, S.M.; Scott, M.G. Electrolytes and Blood Gases. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 604–625. [Google Scholar]
- Freshney, R.I. Defined Media and Supplements. In Culture of Animal Cells—A Manual of Basic Technique and Specialized Applications; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2016; pp. 125–148. [Google Scholar]
- Young, J.A.; VanLennep, E.W. Microscopic Anatomy. In The Morphology of Salivary Glands; Young, J.A., VanLennep, E.W., Eds.; Academic Press Inc.: New York, NY, USA, 1978; pp. 22–48. [Google Scholar]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- Bauer, R.; Plieschnig, J.A.; Finkes, T.; Riegler, B.; Hermann, M.; Schneider, W.J. The Developing Chicken Yolk Sac Acquires Nutrient Transport Competence by an Orchestrated Differentiation Process of Its Endodermal Epithelial Cells. J. Biol. Chem. 2013, 288, 1088–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, J.; Marshall, W. Les Systèmes Mebranaires du Cytoplasme: Structure, Fonction et Circulation des Membranes. In BIologie Cellulaire et Moléculaire de Karp, 4th ed.; Iwasa, J., Marshall, W., Eds.; Deboeck Supérieur: Paris, France, 2016; pp. 257–307. [Google Scholar]
- Smith, M.; Shepherd, J. The Relations Between Yolk and White in the Hen’s Egg. J. Exp. Biol. 1931, 8, 293–311. [Google Scholar]
- Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Mohammadi, P.; Gaudiello, E.; Bonakdar, S.; Solati-Hashjin, M.; Marsano, A.; Aghdami, N.; Scherberich, A.; Baharvand, H.; et al. Facile Fabrication of Egg White Macroporous Sponges for Tissue Regeneration. Adv. Healthc. Mater. 2015, 4, 2281–2290. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charbonneau, A.M.; Tran, S.D. 3D Cell Culture of Human Salivary Glands Using Nature-Inspired Functional Biomaterials: The Egg Yolk Plasma and Egg White. Materials 2020, 13, 4807. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214807
Charbonneau AM, Tran SD. 3D Cell Culture of Human Salivary Glands Using Nature-Inspired Functional Biomaterials: The Egg Yolk Plasma and Egg White. Materials. 2020; 13(21):4807. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214807
Chicago/Turabian StyleCharbonneau, André M., and Simon D. Tran. 2020. "3D Cell Culture of Human Salivary Glands Using Nature-Inspired Functional Biomaterials: The Egg Yolk Plasma and Egg White" Materials 13, no. 21: 4807. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214807