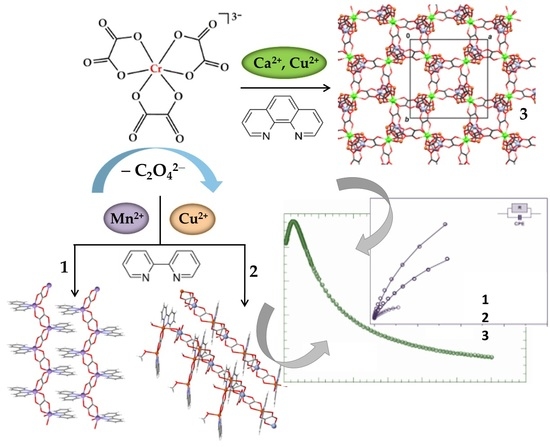
Magnetic and Electrical Behaviors of the Homo- and Heterometallic 1D and 3D Coordination Polymers Based on the Partial Decomposition of the [Cr(C2O4)3]3− Building Block
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Methods
2.2. Synthetic Procedures
2.2.1. Synthesis of {[Mn(bpy)(C2O4)]·1.5H2O}n (1)
2.2.2. Synthesis of {[CrCu3(bpy)3(CH3OH)(H2O)(C2O4)4][Cu(bpy)Cr(C2O4)3]·CH2Cl2·CH3OH·H2O}n (2)
2.2.3. Synthesis of {[CaCr2Cu2(phen)4(C2O4)6]·4CH3CN·2H2O}n (3)
2.3. Single-Crystal X-ray Structural Study
2.4. Magnetic Study
2.5. Electrical Study
3. Results and Discussion
3.1. Synthesis
3.2. Infrared (IR) Spectroscopic Study of Compounds 1–3
3.3. Molecular and Crystal Structures of Compounds 1–3
3.4. Magnetization Study of Compounds 1–3
3.5. Electrical Study of Compounds 1–3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clemente-León, M.; Coronado, E.; Martí-Gastaldoz, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Espallargas, G.M. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The cambridge structural database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, G.; Andruh, M.; Lloret, F.; Julve, M. Bis(oxalato)chromium(III) complexes: Versatile tectons in designing heterometallic coordination compounds. Coord. Chem. Rev. 2011, 255, 161–185. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Ōkawa, H. Design of metal-complex magnets. Syntheses and magnetic properties of mixed-metal assemblies {NBu4[MCr(ox)3]}x (NBu4+ = tetra(n-butyl)ammonium ion; ox2- = oxalate ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Oswald, H.R.; Linden, A.; Ensling, J.; Gütlich, P.; Hauser, A. A polymeric two-dimensional mixed-metal network. crystal structure and magnetic properties of {[P(Ph)4][MnCr(ox)3]}. Inorg. Chim. Acta 1994, 216, 65–73. [Google Scholar] [CrossRef]
- Carling, S.G.; Mathonière, C.; Day, P.; Malik, K.M.A.; Coles, S.J.; Hursthouse, M.B.J. Crystal structure and magnetic properties of the layer ferrimagnet N(n-C5H11)4MnIIFeIII(C2O4)3. J. Chem. Soc. Dalton Trans. 1996, 1839–1843. [Google Scholar] [CrossRef]
- Pellaux, R.; Schmalle, H.W.; Huber, R.; Fischer, P.; Hauss, T.; Ouladdiaf, B.; Decurtins, S. Molecular-Based Magnetism in Bimetallic Two-dimensional oxalate-bridged networks. an X-ray and neutron diffraction study. Inorg. Chem. 1997, 36, 2301–2308. [Google Scholar] [CrossRef]
- Mathonière, C.; Nuttall, C.J.; Carling, S.G.; Day, P. Ferrimagnetic mixed-valency and mixed-metal tris(oxalato)iron(iii) compounds: Synthesis, structure, and magnetism. Inorg. Chem. 1996, 35, 1201–1206. [Google Scholar] [CrossRef]
- Andrés, R.; Gruselle, M.; Malézieux, B.; Verdaguer, M.; Vaissermann, J. Enantioselective Synthesis of optically active polymeric homo- and bimetallic oxalate-bridged networks [M2(ox)3]n. Inorg. Chem. 1999, 38, 4637–4646. [Google Scholar] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Ensling, J.; Gütlich, P. A Concept for the synthesis of 3-dimensional homo- and bimetallic oxalate-bridged networks [M2(ox)3]n. structural, moessbauer, and magnetic studies in the field of molecular-based magnets. J. Am. Chem. Soc. 1994, 116, 9521–9528. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Pellaux, R.; Schneuwly, P.; Hauser, A. Chiral, Three-dimensional supramolecular compounds: homo- and bimetallic oxalate- and 1, 2-dithiooxalate-bridged networks. a structural and photophysical study. Inorg. Chem. 1996, 35, 1451–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decurtins, S.; Stoeckli-Evans, H.; Wilson, C.; Yufit, D.; Howard, J.A.K.; Capelli, S.C.; Hauser, A. A thermal spin transition in [Co(bpy)3][LiCr(ox)3] (ox = C2O42−; bpy = 2,2’-bipyridine). Chem.-Eur. J. 2000, 6, 361–368. [Google Scholar]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Rational designs for highly proton-conductive metal−organic frameworks. J. Am. Chem. Soc. 2009, 131, 9906–9907. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Proton conductivity control by ion substitution in a highly proton-conductive metal–organic framework. J. Am. Chem. Soc. 2014, 136, 13166–13169. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Ōkawa, H.; Shigematsu, A.; Ohba, M.; Yamada, T.; Kitagawa, H. Promotion of low-humidity proton conduction by controlling hydrophilicity in layered metal–organic frameworks. J. Am. Chem. Soc. 2012, 134, 5472–5475. [Google Scholar] [CrossRef]
- Lim, D.-W.; Sadakiyo, M.; Kitagawa, H. Proton transfer in hydrogen-bonded degenerate systems of water and ammonia in metal–organic frameworks. Chem. Sci. 2019, 10, 16–33. [Google Scholar]
- Maxim, C.; Ferlay, S.; Tokoro, H.; Ohkoshi, S.-I.; Train, C. Atypical stoichiometry for a 3D bimetallic oxalate-based long-range ordered magnet exhibiting high proton conductivity. Chem. Commun. 2014, 50, 5629–5632. [Google Scholar] [CrossRef]
- Ōkawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-bridged bimetallic complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII, CoII; NH(prol)3+ = Tri(3-hydroxypropyl)ammonium) exhibiting coexistent ferromagnetism and proton conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Train, C.; Gontard, G.; Boubekeur, K.; Fabelo, O.; Liu, H.; Dkhil, B.; Lloret, F.; Nakagawa, K.; Tokoro, H.; et al. High proton conduction in a chiral ferromagnetic metal–organic quartz-like framework. J. Am. Chem. Soc. 2011, 133, 15328–15331. [Google Scholar] [CrossRef] [PubMed]
- Habjanič, J.; Jurić, M.; Popović, J.; Molčanov, K.; Pajić, D. A 3D oxalate-based network as a precursor for the comn2o4 spinel: Synthesis and structural and magnetic studies. Inorg. Chem. 2014, 53, 9633–9643. [Google Scholar] [CrossRef] [PubMed]
- Jurić, M.; Pajić, D.; Žilić, D.; Rakvin, B.; Molčanov, K.; Popović, J. Magnetic order in a novel 3D oxalate-based coordination polymer {[Cu(bpy)3][Mn2(C2O4)3]·H2O}n. Dalton Trans. 2015, 44, 20626–20635. [Google Scholar]
- Žilić, D.; Molčanov, K.; Jurić, M.; Habjanič, J.; Rakvin, B.; Krupskaya, Y.; Kataev, V.; Wurmehl, S.; Büchner, B. 3D oxalate-based coordination polymers: Relationship between structure, magnetism and color, studied by high-field esr spectroscopy. Polyhedron 2017, 126, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Kanižaj, L.; Molčanov, K.; Torić, F.; Pajić, D.; Lončarić, I.; Šantić, A.; Jurić, M. Ladder-like [CrCu] coordination polymers containing unique bridging modes of [Cr(C2O4)3]3− and Cr2O72−. Dalton Trans. 2019, 48, 7891–7898. [Google Scholar] [CrossRef]
- Kanižaj, L.; Androš Dubraja, L.; Torić, F.; Pajić, D.; Molčanov, K.; Wenger, E.; Jurić, M. Dimensionality controlled by light exposure: 1D versus 3D oxalate-bridged [CuFe] coordination polymers based on an [Fe(C2O4)3]3− metallotecton. Inorg. Chem. Front. 2019, 6, 3327–3335. [Google Scholar] [CrossRef]
- Brauer, G. Handbuch der präparativen anorganischen Chemie. F. Enke: Stuttgart, Germany, 1981. [Google Scholar]
- Castillo, O.; Luque, A.; Iglesias, S.; Guzm, C.; Rom, P. A novel one-dimensional oxalato-bridged copper (II) complex with 2,2’-bipyridine. Inorg. Chem. Commun. 2001, 4, 640–642. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlis P.R.O.; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program. PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP3 for Windows-a version of ORTEP III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A. Voronoi–dirichlet polyhedra in crystal chemistry: Theory and applications. Crystallogr. Rev. 2004, 10, 249–318. [Google Scholar] [CrossRef]
- Spingler, B.; Schnidrig, S.; Todorova, T.; Wild, F. Some thoughts about the single crystal growth of small molecules. CrystEngComm 2012, 14, 751–757. [Google Scholar] [CrossRef]
- Androš Dubraja, L.; Jurić, M.; Torić, F.; Pajić, D. The influence of metal centres on the exchange interaction in heterometallic complexes with oxalate-bridged cations. Dalton Trans. 2017, 46, 11748–11756. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pereira, C.L.M.; Pinheiro, C.B.; Lloret, F.; Julve, M. Towards oxalate-bridged iron (II), cobalt (II), nickel(II) and zinc(II) complexes through oxotris(oxalato)niobate(V): An open air non-oxidizing synthetic route. Inorg. Chem. Front. 2018, 5, 1294–1306. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pereira, C.L.M.; Pinheiro, C.B.; Lloret, F.; Julve, M. Oxotris (oxalate)niobate(V): An oxalate delivery agent in the design of building blocks. J. Coord. Chem. 2018, 71, 707–724. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley: New York, NY, USA, 2009. [Google Scholar]
- Deguenon, D.; Bernardinelli, G.; Tuchagues, J.P.; Castan, P. Molecular crystal structure and magnetic properties of (croconato)-and (oxalato) manganese(II) complexes. Inorg. Chem. 1990, 29, 3031–3037. [Google Scholar] [CrossRef]
- Yu, J.-H.; Hou, Q.; Bi, M.-H.; Lü, Z.-L.; Zhang, X.; Qu, X.-J.; Lu, J.; Xu, J.-Q. Structure characterization of several oxalate-bridged transition-metal coordination polymers. J. Mol. Struct. 2006, 800, 69–73. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvares, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Jurić, M.; Planinić, P.; Brničvić, N.; Milić, D.; Matković-Čalogović, D.; Pajić, D.; Zadro, K. New heterometallic (CuII and CrIII) complexes – first crystal structure of an oxalate-bridged ferromagnetically coupled [CuIICrIIICuII] system. Eur. J. Inorg. Chem. 2006, 2701–2710. [Google Scholar] [CrossRef]
- Marinescu, G.; Diana Visinescu, D.; Cucos, A.; Andruh, M.; Journaux, Y.; Kravtsov, V.; Simonov, Y.A.; Lipkowski, J. Oxalato-bridged [CuIICrIII] and [MnIICrIII] binuclear complexes: Synthesis, crystal structures, magnetic and EPR investigations. Eur. J. Inorg. Chem. 2004, 2914–2922. [Google Scholar] [CrossRef]
- Ohba, M.; Tamaki, H.; Matsumoto, N.; Okawa, H. Oxalate-bridged dinuclear chromium(III)-M(II) (M = copper, nickel, cobalt, iron, manganese) complexes: Synthesis, structure, and magnetism. Inorg. Chem. 1993, 32, 5385–5390. [Google Scholar] [CrossRef]
- Andruh, M.; Melanson, R.; Stager, C.V.; Rochon, F.D. [Cr(bipy)(C2O4)2]−—A versatile building block for the design of heteropolymetallic systems. 2. Syntheses and crystal structures of CuCr2(bipy)2(µ-C2O4)2(C2O4)2(H2O)2·1½H2O and (AgCr(bipy)(µ-C2O4)2(H2O)2)2 and magnetic properties of the copper(II) derivative. Inorg. Chim. Acta 1996, 251, 309–317. [Google Scholar]
- Coronado, E.; Giménez, M.C.; Gómez-García, C.J.; Romer, F.M. Synthesis, crystal structure and magnetic propertiesof [Cr2Cu2(bpy)4(ox)5]·2H2O. An oxalato-bridged heterometallic tetramer. Polyhedron 2003, 22, 3115–3122. [Google Scholar] [CrossRef]
- Santiago Reinoso, S.; Vitoria, P.; San Felices, L.; Lezama, L.; Gutiérrez-Zorrilla, J.M. µ-Oxalato-κ4O1,O2:O1′,O2′-bis[aqua(2,2′-bipyridyl-κ2N,N′)copper(II)] bis(hydrogen squarate). Acta Crystallogr. 2005, E61, m1677–m1679. [Google Scholar]
- Androš, L.; Jurić, M.; Popović, J.; Pajić, D.; Zadro, K.; Molčanov, K.; Žilić, D.; Planinić, P. 1D heterometallic oxalate compounds as precursors for mixed Ca–Cr oxides–synthesis, structures, and magnetic studies. Eur. J. Inorg. Chem. 2014, 5703–5713. [Google Scholar] [CrossRef]
- Khan, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- Caputo, R.E.; Willet, R.D. Crystal structure and magnetic susceptibility of (CH3)2NH2MnCl3 (DMMC): A low-symmetry analog of (CH3)4NMnCl3 (TMMC). Phys. Rev. B 1976, 13, 3956. [Google Scholar] [CrossRef]
- Novosel, N.; Žilić, D.; Pajić, D.; Jurić, M.; Perić, B.; Zadro, K.; Rakvin, B.; Planinić, P. EPR and magnetization studies on single crystals of a heterometallic(CuII and CrIII) complex: Zero-field splitting determination. Solid State Sci. 2008, 10, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- García-Terán, J.P.; Castillo, O.; Luque, A.; García-Couceiro, U.; Román, P.; Lloret, F. One-Dimensional Oxalato-Bridged Cu(II), Co(II), and Zn(II) Complexes with Purine and Adenine as Terminal Ligands. Inorg. Chem. 2004, 43, 5761–5770. [Google Scholar] [CrossRef]
- Lah, N.; Clerac, R. Cu(II) oxalate coordination polymer based on 3-pyridinepropanol bridging ligand: Synthesis, characterization and magnetic properties. Inorg. Chem. Commun. 2014, 41, 62–64. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Torić, F.; Pavlović, G.; Pajić, D.; Cindrić, M.; Zadro, K. Tetranuclear Ni4 cubane complexes with high χT maxima: Magneto-structural analysis. CrystEngComm 2018, 20, 3917–3927. [Google Scholar] [CrossRef]
- Jurić, M.; Androš Dubraja, L.; Pajić, D.; Torić, F.; Zorko, A.; Ozarowski, A.; Despoja, V.; Lafargue-Dit-Hauret, W.; Rocquefelte, X. Experimental and Theoretical Investigation of the Anti-Ferromagnetic Coupling of CrIII Ions through Diamagnetic −O−NbV−O− Bridges. Inorg. Chem. 2017, 56, 6879–6889. [Google Scholar]
- Oliveira, W.X.C.; Pereira, C.L.M.; Pinheiro, C.B.; Cano, J.; Lloret, F.; Julve, M. Relatively strong intramolecular antiferromagnetic coupling in a neutral CrIII2NbV2 heterobimetallic molecular square. Chem. Commun. 2015, 51, 11806–11809. [Google Scholar] [CrossRef]












| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C12H9MnN2O5 | C57H46Cl2Cr2Cu4N8O32 | C68H48CaCr2Cu2N12O26 |
| Formula wt./g mol−1 | 324.15 | 1784.12 | 1720.36 |
| Colour | yellow | green | dark green |
| Crystal dimensions/mm | 0.23 × 0.14 × 0.09 | 0.80 × 0.50 × 0.20 | 0.25 × 0.15 × 0.05 |
| Space group | C2/c | P | P41212 |
| a/Å | 14.3981(3) | 8.7537(2) | 16.1956(1) |
| b/Å | 12.7019(2) | 18.5551(6) | 16.1956(1) |
| c/Å | 16.5031(5) | 21.4099(7) | 26.5448(2) |
| α/° | 90 | 72.907(3) | 90 |
| β/° | 118.536(3) | 81.372(2) | 90 |
| γ/° | 90 | 85.676(2) | 90 |
| Z | 8 | 2 | 4 |
| V/Å3 | 2651.49(13) | 3284.50(18) | 6962.63(10) |
| Dcalc/g cm−3 | 1.624 | 1.802 | 1.641 |
| μ/mm−1 | 8.358 | 5.677 | 4.651 |
| F(000) | 1312 | 1796 | 437 |
| Θ range/° | 6.42–75.85 | 3.74–75.98 | 3.19–75.28 |
| T/K | 293(2) | 100(2) | 293(2) |
| Range of h, k, l | −18 < h < 16; | −10 < h < 10 | −20 < h < 13 |
| −15 < k < 15; | −23 < k < 23 | −20 < k < 19 | |
| −16 < l < 20 | −26 < l < 26 | −26 < l < 33 | |
| Reflections collected | 6468 | 34327 | 22852 |
| Independent reflections | 2705 | 13553 | 7164 |
| Observed reflections (I ≥ 2σ) | 2448 | 12642 | 6857 |
| Rint | 0.0230 | 0.0326 | 0.0377 |
| R, wR [I ≥ 2σ] | 0.0321 | 0.0390 | 0.0351 |
| wR [I ≥ 2σ] | 0.0876 | 0.1057 | 0.0937 |
| Goodness-of-fit | 1.033 | 1.048 | 1.042 |
| H atom treatment | Mixed | Mixed | Mixed |
| No. of parameters, restraints | 200, 2 | 974, 21 | 505, 6 |
| Δρmax, Δρmin, Δρrms (eÅ−3) | 0.201; −0.276; 0.045 | 1.249; −1,574; 0.082 | 0.299; −0.432; 0.052 |
| Compound | Bidentate Oxalate Group | Bis(bidentate) Oxalate Group | ||||
|---|---|---|---|---|---|---|
| νas(CO) | νs(CO) | δ(OCO) | νas(CO) | νs(CO) | δ(OCO) | |
| 1 | – | – | – | 1673 | 1310 | 793 |
| 2 | 1702, 1679, 1634 | 1389, 1252 | 814 | 1664 | 1293 | 773 |
| 3 | – | – | – | 1664 | 1282 | 781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanižaj, L.; Šenjug, P.; Pajić, D.; Pavić, L.; Molčanov, K.; Jurić, M. Magnetic and Electrical Behaviors of the Homo- and Heterometallic 1D and 3D Coordination Polymers Based on the Partial Decomposition of the [Cr(C2O4)3]3− Building Block. Materials 2020, 13, 5341. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13235341
Kanižaj L, Šenjug P, Pajić D, Pavić L, Molčanov K, Jurić M. Magnetic and Electrical Behaviors of the Homo- and Heterometallic 1D and 3D Coordination Polymers Based on the Partial Decomposition of the [Cr(C2O4)3]3− Building Block. Materials. 2020; 13(23):5341. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13235341
Chicago/Turabian StyleKanižaj, Lidija, Pavla Šenjug, Damir Pajić, Luka Pavić, Krešimir Molčanov, and Marijana Jurić. 2020. "Magnetic and Electrical Behaviors of the Homo- and Heterometallic 1D and 3D Coordination Polymers Based on the Partial Decomposition of the [Cr(C2O4)3]3− Building Block" Materials 13, no. 23: 5341. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13235341