Photocurable Polymeric Blends for Surgical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blending and Crosslinkg
2.2.1. Lactic Acid Oligomers Synthesis
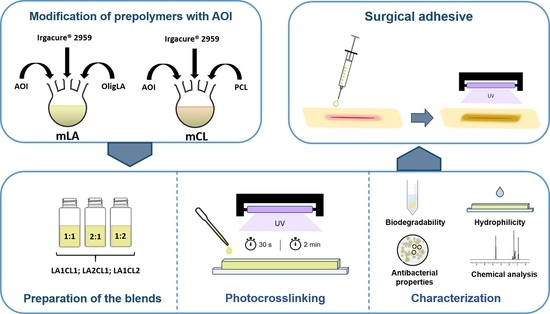
2.2.2. Modification of Prepolymers with AOI
2.2.3. Preparation of Blends and Photocrosslinking
- LA1CL1: mLA and mCL in 1:1 ratio;
- LA2CL1: mLA and mCL in a 2:1 ratio;
- LA1CL2: mLA and mCL in a 1:2 ratio.
2.3. Characterization Techniques
2.3.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.2. Rheological Studies
2.3.3. Gel Content
2.3.4. Hydrolytic Degradation
2.3.5. Dynamic Contact Angles Measurements
2.3.6. Thermal Analysis
2.3.7. Cell Growth and Proliferation
2.3.8. Antimicrobial Activity
3. Results and Discussion
3.1. Synthesis
3.2. 1H NMR Analysis
3.3. Rheological Studies
3.4. Dynamic Contact Angles
3.5. Gel Content
3.6. Hydrolytic Degradation
3.7. Thermal Properties
3.8. Characterization of the Crosslinked Polymeric Blends’ Biological Properties
3.8.1. Cell Growth and Proliferation
3.8.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doulabi, A.H.; Mequanint, K.; Mohammadi, H. Blends and nanocomposite biomaterials for articular cartilage tissue engineering. Materials 2014, 7, 5327–5355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cernadas, T.M.; Gonçalves, F.A.M.M.; Alves, P.; Miguel, S.P.; Cabral, C.; Correia, I.J.; Ferreira, P. Preparation of biodegradable functionalized polyesters aimed to be used as surgical adhesives. Eur. Polym. J. 2019, 117, 442–454. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. Blends and composites based on cellulose and natural polymers. In Biodegradable Polymer Blends and Composites from Renewable Resources; Long, Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 129–161. ISBN 9780470146835. [Google Scholar]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Santos, J.M.C.; Marques, D.S.; Alves, P.; Correia, T.R.; Correia, I.J.; Baptista, C.M.S.G.; Ferreira, P. Synthesis, functionalization and characterization of UV-curable lactic acid based oligomers to be used as surgical adhesives. React. Funct. Polym. J. 2015, 94, 43–54. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(D,L-lactic acid)/poly(ε-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Mulinti, P.; Brooks, J.E.; Lervick, B.; Pullan, J.E.; Brooks, A.E. 10-Strategies to improve the hemocompatibility of biodegradable biomaterials. In Hemocompatibility of Biomaterials for Clinical Applications; Woodhead Publishing: Cambridge, UK, 2018; pp. 253–278. [Google Scholar]
- Albertsson, A.C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. Degrad. Aliphatic Polyesters 2002, 157, 1–40. [Google Scholar]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Cernadas, T.; Morgado, S.; Alves, P.; Gonçalves, F.A.M.M.; Correia, T.R.; Correia, I.J.; Ferreira, P. Preparation of functionalized poly(caprolactone diol)/castor oils blends to be applied as photocrosslinkable tissue adhesives. J. Appl. Polym. Sci. 2020, 1–14. [Google Scholar] [CrossRef]
- Cernadas, T.; Santos, M.; Gonçalves, F.A.M.M.; Alves, P.; Correia, T.R.; Correia, I.J.; Ferreira, P. Functionalized polyester-based materials as UV curable adhesives. Eur. Polym. J. 2019, 120, 109196. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Wu, S.-C.; Chen, H.; Tsai, L.-L.; Tzeng, J.-J.; Lin, C.-H.; Lin, Y.-M. Synthesis and Characterization of Polycaprolactone-Based Polyurethanes for the Fabrication of Elastic Guided Bone Regeneration Membrane. Biomed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, P.; Santos, M.; Mendes, S.; Miguel, S.P.; de Sá, K.D.; Cabral, C.S.D.; Correia, I.J.; Ferreira, P. Photocrosslinkable nanofibrous asymmetric membrane designed for wound dressing. Polymers 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Travassos, D.R.S.; Santos, J.M.C.; Baptista, C.M.S.G.; Marques, D.S.; Ferreira, P.; Gil, M.H.; Ribeiro, M.P.; Correia, I.J.; Miguel, S.P. Engineering star-shaped lactic acid oligomers to develop novel functional adhesives. J. Mater. Res. 2018, 33, 1463–1474. [Google Scholar]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(d,l-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- Malik, M.; Kaur, R. Mechanical and thermal properties of castor oil–based polyurethane adhesive: Effect of TiO2 filler. Adv. Polym. Technol. 2018, 37, 21637. [Google Scholar] [CrossRef]
- Valero, M.F.; Ortegón, Y. Polyurethane elastomers-based modified castor oil and poly(ε-caprolactone) for surface-coating applications: Synthesis, characterization, and in vitro degradation. J. Elastomers Plast. 2013, 47, 360–369. [Google Scholar] [CrossRef]
- Sabnis, A.; Rahimi, M.; Chapman, C.; Nguyen, K.T. Cytocompatibility studies of an in situ photopolymerized thermoresponsive hydrogel nanoparticle system using human aortic smooth muscle cells. J. Biomed. Mater. Res. A 2009, 91, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/Aloe Vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.S.; Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Tsao, H.K.; Sheng, Y.J.; Chen, S.; Jiang, S. Strong repulsive forces between protein and oligo (ethylene glycol) self-assembled monolayers: A molecular simulation study. Biophys. J. 2005, 89, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Ray, B.; Daeschel, M. Food Biopreservatives of Microbial Origin, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]










| Adhesive Blends | UV Irradiation Time (s) | Gel Content (%) |
|---|---|---|
| LA1CL1 | 30 | 64.2 ± 3.40 |
| 120 | 77.6 ± 0.27 | |
| LA1CL2 | 30 | 68.9 ± 0.45 |
| 120 | 79.5 ± 0.31 | |
| LA2CL1 | 30 | 47.8 ± 1.78 |
| 120 | 68.1 ± 0.14 |
| 1st Stage | 2nd Stage | 3rd Stage | ||||||
|---|---|---|---|---|---|---|---|---|
| Tmax1 (°C) | ΔW1 (wt %) | Tmax2 (°C) | ΔW2 (wt %) | Tmax3 (°C) | ΔW3 (wt %) | T50 wt % (°C) | Residue at 500 °C (wt %) | |
| LA1CL1 | 209.32 | 28.87 | 347.00 | 70.82 | 404.91 | 90.08 | 272.69 | 1.34 |
| LA1CL2 | 207.41 | 21.92 | 357.99 | 66.00 | 405.57 | 87.40 | 319.50 | 1.17 |
| LA2CL1 | 206.91 | 31.83 | 333.83 | 70.65 | 400.09 | 89.53 | 247.79 | 1.55 |
| LA1CL1-30s | 206.30 | 23.00 | 320.66 | 57.02 | 406.49 | 85.00 | 301.83 | 2.21 |
| LA1CL2-30s | 216.83 | 16.52 | 348.44 | 54.78 | 410.88 | 83.30 | 338.78 | 2.02 |
| LA2CL1-30s | 213.18 | 20.51 | 312.46 | 62.36 | 405.70 | 85.58 | 274.41 | 1.99 |
| LA1CL1-2min | 194.07 | 21.45 | 330.04 | 60.39 | 404.24 | 84.65 | 301.66 | 2.29 |
| LA1CL2-2min | 209.76 | 15.67 | 342.45 | 52.75 | 407.79 | 81.94 | 337.04 | 2.23 |
| LA2CL1-2min | 203.30 | 25.90 | 300.66 | 56.05 | 408.31 | 84.93 | 282.24 | 2.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernadas, T.; Santos, M.; Miguel, S.P.; Correia, I.J.; Alves, P.; Ferreira, P. Photocurable Polymeric Blends for Surgical Application. Materials 2020, 13, 5681. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13245681
Cernadas T, Santos M, Miguel SP, Correia IJ, Alves P, Ferreira P. Photocurable Polymeric Blends for Surgical Application. Materials. 2020; 13(24):5681. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13245681
Chicago/Turabian StyleCernadas, Teresa, Marta Santos, Sónia P. Miguel, Ilídio J. Correia, Patrícia Alves, and Paula Ferreira. 2020. "Photocurable Polymeric Blends for Surgical Application" Materials 13, no. 24: 5681. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13245681