Dentin Microhardness and Sealer Bond Strength to Root Dentin are Affected by Using Bioactive Glasses as Intracanal Medication
Abstract
:1. Introduction
2. Materials and Methods
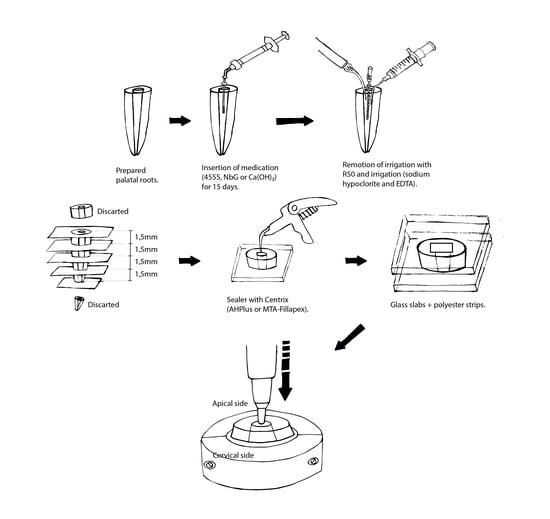
2.1. Preparation of the Roots
2.2. Treatment of the Samples and Dentin Microhardness (MH) Analysis
2.3. Treatment of the Samples and Sealers’ Bond Strength to Dentin Analysis (BS)
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoldas, O.; Dogan, C.; Seydaoglu, G. The effect of two different calcium hydroxide silicate-based materials on collagen matrix integrity of mineralized dentin. J. Endod. 2012, 38, 829–833. [Google Scholar]
- Yilmaz, S.; Dumani, A.; Yoldas, O. The effect of antibiotic pastes on microhardness of dentin. Dent. Traumatol. 2016, 32, 27–31. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Farik, B.; Munksgaard, E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 2002, 18, 134–137. [Google Scholar] [CrossRef]
- Yassen, G.H.; Vail, M.M.; Chu, T.G.; Platt, J.A. The effect of medicaments used in endodontic regeneration on root fracture and microhardness of radicular dentine. Int. Endod. J. 2013, 46, 688–695. [Google Scholar] [CrossRef]
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Ghasemi, N.; Shahi, S.; Lotfi, M.; Froughreyhani, M.; Milani, A.S.; Bahari, M. Effect of blood contamination on the retention characteristics of two endodontic biomaterials in simulated furcation perforations. J. Endod. 2013, 39, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Marquezan, F.K.; Kopper, P.M.P.; Dullius, A.I.S.; Ardenghi, D.M.; Grazziotin-Soares, R. Effect of the blood contamination on the push-out bond strength of Calcium Silicate Cements. Braz. Dent. J. 2018, 29, 1891–1894. [Google Scholar] [CrossRef] [Green Version]
- Kinney, J.H.; Habelitz, S.; Marshall, S.J.; Marshall, G.W. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J. Dent. Res. 2003, 82, 957–961. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jiang, T.; Sauro, S.; Wang, Y.; Thompson, I.; Watson, T.F.; Sa, Y.; Xing, W.; Shen, Y.; Haapasalo, M. Dentine remineralization induced by two bioactive glasses developed for air abrasion purposes. J. Dent. 2011, 39, 746–756. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Habelitz, S.; Marshall, S.J.; Marshall, G.W. Mechanical recovery of dentin following remineralization in vitro—An indentation study. J. Biomech. 2011, 44, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Zehnder, M.; Söderling, E.; Salonen, J.; Waltimo, T. Preliminary evaluation of bioactive glass S53P4 as an endodontic medication in vitro. J. Endod. 2004, 30, 220–224. [Google Scholar]
- Zehnder, M.; Waltimo, N.; Sener, B.; Söderling, E. Dentin enhances the effectiveness of bioactive glass S53P4 against a strain of Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Marending, M.; Stark, W.J.; Brunner, T.J.; Fischer, J.; Zehnder, M. Comparative assessment of time-related bioactive glass and calcium hydroxide effects on mechanical properties of human root dentin. Dent Traumatol. 2009, 25, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. Symp. 1971, 2, 117–141. [Google Scholar] [CrossRef]
- Wilson, J.; Noletti, D. Bonding of soft tissues to Bioglass®. In Handbook of Bioactive Ceramics; Yamamuro, T., Hench, L.L., Wilson, J., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 283–302. [Google Scholar]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [Green Version]
- Sene, F.F.; Martinelli, J.R.; Gomes, L. Synthesis and characterization of niobium phosphate glasses containing barium and potassium. J. Non-Cryst. Solids. 2004, 348, 30–37. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Martinelli, J.R.; Bauer, J.; Haapasalo, M.; Shen, Y.; Bradaschia-Correa, V.; Manso, A.P.; Gavini, G. Micropush-out dentine bond strength of a new gutta-percha and niobium phosphate glass composite. Int. Endod. J. 2015, 48, 4514–4559. [Google Scholar] [CrossRef]
- Leitune, V.C.; Collares, F.M.; Takimi, A.; Lima, G.B.; Petzhold, C.L.; Bergmann, C.P. Niobium pentoxide as a novel filler for dental adhesive resin. J. Dent. 2013, 41, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitune, V.C.; Takimi, A.; Collares, F.M.; Santos, P.D.; Provenzi, C.; Bergmann, C.P. Niobium pentoxide as a new filler for methacrylate-based root canal sealers. Int. Endod. J. 2013, 46, 205–210. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Wang, Z.; Shen, Y.; Gavini, G.; Martinelli, J.R.; Manso, A.; Haapasalo, M. Comparative analyses of ion release, pH and multispecies biofilm formation between conventional and bioactive gutta-percha. Int. Endod. J. 2016, 49, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.N.; Freire, L.G.; Carvalho, A.P.; Duarte, M.A.; Bauer, J.; Gavini, G. Ions Release and pH of Calcium Hydroxide-, Chlorhexidine- and Bioactive Glass-Based Endodontic Medicaments. Braz. Dent. J. 2016, 27, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.N.; Grazziotin-Soares, R.; de Miranda Candeiro, G.T.; Gallego Martinez, L.; de Souza, J.P.; Santos Oliveira, P.; Bauer, J.; Gavini, G. Micro Push-out Bond Strength and Bioactivity Analysis of a Bioceramic Root Canal Sealer. Iran. Endod. J. 2017, 12, 343–348. [Google Scholar] [PubMed]
- Carvalho, C.N.; Bauer, J.; Ferrari, P.H.; Souza, S.F.; Soares, S.P.; Loguercio, A.D.; Bombana, A.C. Influence of calcium hydroxide intracanal medication on bond strength of two endodontic resin-based sealers assessed by micropush-out test. Dent. Traumatol. 2013, 29, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S. Adhesive dentistry and endodontics. Part 2: Bonding in the root canal system-The promise and the problems: A review. J. Endod. 2006, 32, 1125–1134. [Google Scholar]
- Souza, S.F.C.; Bombana, A.C.; Francci, C.; Gonçalves, F.; Castellan, C.; Braga, R.R. Polymerization stress, flow and dentine bond strength of two resin-based root canal sealers. Int. Endod. J. 2009, 42, 867–873. [Google Scholar] [CrossRef]
- Angelus. MTA Fillapex Endodontic sealer. Scientific profile. Available online: http://www.angelusdental.com/img/arquivos/mta_fillapex_technical_profile_download.pdf (access on 1 September 2019).
- Carvalho, N.K.; Prado, M.C.; Senna, P.M.; Neves, A.A.; Souza, E.M.; Fidel, S.R.; Sassone, L.M.; Silva, E.J.N.L. Do smear-layer removal agents affect the push-out bond strength of calcium silicate-based endodontic sealers? Int. Endod. J. 2017, 50, 612–619. [Google Scholar] [CrossRef]

| Intracanal Medication | Dentinal Microhardness | ||
|---|---|---|---|
| Baseline (day 0–no medication) | After Medication (day 15) | Variation (%) | |
| NbG | 34.7 ± 7.1 B | 45.2 ± 11.2 A | +37.7 ± 37.6 a |
| 45S5 | 35.4 ± 9.9 B | 45.3 ± 8.7 A | +38.7 ± 48.8 a |
| Ca(OH)2 | 40.8 ± 4.9 A | 29.5 ± 6.7 B | −23.1 ± 16.1 b |
| Intracanal Medication (15 days) | |||||
|---|---|---|---|---|---|
| NbG | 45S5 | Ca(OH)2 | Control (No Medication) | ||
| Sealer | AH Plus® | 4.51a (± 2.6) | 7.14a (± 3.9) | 7.13a (± 2.7) | 11.33b (± 3.5) |
| MTA Fillapex® | 0.33 (± 1.68) | 0.15 (± 0.5) | 0.97 (± 0.7) | 0.38 (± 0.2) | |
| Groups | Adhesive (%) | Cohesive (%) | Mixed (%) |
|---|---|---|---|
| NbG + AH | 10.15 | 71.9 | 18.65 |
| 45S5 + AH | 5.7 | 70 | 24.3 |
| Ca(OH)2 + AH | 0 | 79.5 | 20.5 |
| no medication + AH | 0 | 71 | 29.0 |
| NbG + Fillapex | 0 | 83 | 17 |
| 45S5 + Fillapex | 0 | 85 | 15 |
| Ca(OH)2 + Fillapex | 0 | 79 | 21 |
| no medication + Fillapex | 0 | 84 | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grazziotin-Soares, R.; Dourado, L.G.; Gonçalves, B.L.L.; Ardenghi, D.M.; Ferreira, M.C.; Bauer, J.; Carvalho, C.N. Dentin Microhardness and Sealer Bond Strength to Root Dentin are Affected by Using Bioactive Glasses as Intracanal Medication. Materials 2020, 13, 721. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13030721
Grazziotin-Soares R, Dourado LG, Gonçalves BLL, Ardenghi DM, Ferreira MC, Bauer J, Carvalho CN. Dentin Microhardness and Sealer Bond Strength to Root Dentin are Affected by Using Bioactive Glasses as Intracanal Medication. Materials. 2020; 13(3):721. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13030721
Chicago/Turabian StyleGrazziotin-Soares, Renata, Letícia Gomes Dourado, Bruna Lais Lins Gonçalves, Diego Machado Ardenghi, Meire Coelho Ferreira, José Bauer, and Ceci Nunes Carvalho. 2020. "Dentin Microhardness and Sealer Bond Strength to Root Dentin are Affected by Using Bioactive Glasses as Intracanal Medication" Materials 13, no. 3: 721. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13030721