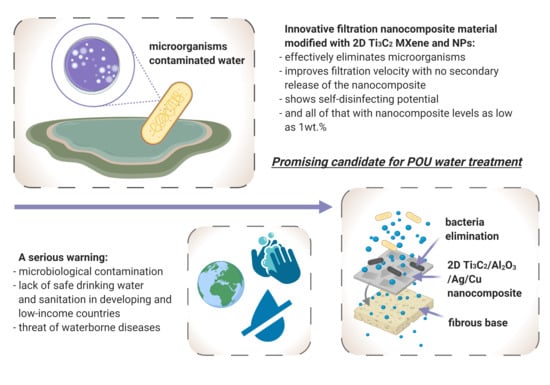
Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Nanocomposites
2.2. Development of the Modified Polypropylene Filter Fabric
2.3. Characterization of the Morphology and Chemical Composition of MXene-Based Composites and Surface-Modified Polypropylene Materials
2.4. The Evaluation of Antimicrobial Properties of Nanocomposites
2.5. Filtration Tests
- Pristine (unmodified) polypropylene fabrics,
- Polypropylene fabrics modified with Ti3C2/Al2O3/Ag/Cu (8 wt %),
- Polypropylene filter subjected to surface oxidation after nanocomposite enrichment with TiO2 crystals (labeled as o-Ti3C2/Al2O3/Ag/Cu).
3. Results
3.1. Structure and Properties of Modified Polypropylene Materials
3.2. Filtration Process
3.3. Filtrate Parameters
3.4. UV-Vis Study of The Filtrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pooi, C.K.; Ng, H.Y. Review of low-cost point-of-use water treatment systems for developing communities. npj Clean Water 2018, 1. [Google Scholar] [CrossRef]
- Bitton, G. Microbiology of Drinking Water: Production and Distribution; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Gleick, P.H. Water and terrorism. Water Policy 2006, 8, 481–503. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Africa. Available online: https://www.afro.who.int/health-topics/water (accessed on 9 November 2020).
- Sobsey, M.D.; Stauber, C.E.; Casanova, L.M.; Brown, J.M.; Elliott, M.A. Response to comment on “Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world”. Environ. Sci. Technol. 2009, 43, 970–971. [Google Scholar] [CrossRef]
- Heidarpour, F.; Wan Ab Karim Ghani, W.; Ahmadun, F.-R.; Sobri, S.; Zargar, M.; Mozafari, M. Nano silver-coated polypropylene water filter: II. evaluation of antimicrobial efficiency. Dig. J. Nanomater. Biostructures 2010, 5, 797–804. [Google Scholar]
- Heidarpour, F.; Wan Ab Karim Ghani, W.A.; Fakhru’L-Razi, A.; Sobri, S.; Heydarpour, V.; Zargar, M.; Mozafari, M.R. Complete removal of pathogenic bacteria from drinking water using nano silver-coated cylindrical polypropylene filters. Clean Technol. Environ. Policy 2011, 13, 499–507. [Google Scholar] [CrossRef]
- Srinivasan, N.R.; Shankar, P.A.; Bandyopadhyaya, R. Plasma treated activated carbon impregnated with silver nanoparticles for improved antibacterial effect in water disinfection. Carbon N. Y. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Oyanedel-Craver, V. Comparison of the bacterial removal performance of silver nanoparticles and a polymer based quaternary amine functiaonalized silsesquioxane coated point-of-use ceramic water filters. J. Hazard. Mater. 2013, 260, 272–277. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Ortiz Balbuena, J.; Tutor De Ureta, P.; Rivera Ruiz, E.; Mellor Pita, S. Enfermedad de Vogt-Koyanagi-Harada. Med. Clin. (Barc) 2016, 146, 93–94. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti 3AlC 2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguib, M.; Presser, V.; Lane, N.; Tallman, D.; Gogotsi, Y.; Lu, J.; Hultman, L.; Barsoum, M.W. Synthesis of a new nanocrystalline titanium aluminum fluoride phase by reaction of Ti 2AlC with hydrofluoric acid. RSC Adv. 2011, 1, 1493–1499. [Google Scholar] [CrossRef]
- Naguib, M.; Presser, V.; Tallman, D.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. On the topotactic transformation of Ti2AlC into a Ti-C-O-F cubic phase by heating in molten lithium fluoride in air. J. Am. Ceram. Soc. 2011, 94, 4556–4561. [Google Scholar] [CrossRef] [Green Version]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. On the Chemical Diversity of the MAX Phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, G.; Zuo, X.; Tang, H.; Yang, Q.; Li, G. Computational studies on the structural, electronic and optical properties of graphene-like MXenes (M2CT2, M = Ti, Zr, Hf; T = O, F, OH) and their potential applications as visible-light driven photocatalysts. J. Mater. Chem. A 2016, 4, 12913–12920. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, J.; Zou, G.; Peng, Q.; Du, Q.; Jiao, T.; Xiang, J. Efficient phosphate sequestration for water purification by unique sandwich-like MXene/magnetic iron oxide nanocomposites. Nanoscale 2016, 8, 7085–7093. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water. ACS Sustain. Chem. Eng. 2017, 5, 11481–11488. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Van der Bruggen, B. An MXene-based membrane for molecular separation. Environ. Sci. Nano 2020, 7, 1289–1304. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti 2 C and Ti 3 C 2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria. J. Mater. Eng. Perform. 2019, 28, 1272–1277. [Google Scholar] [CrossRef]
- Arabi Shamsabadi, A.; Sharifian, M.; Anasori, B.; Soroush, M. Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene Nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 16586–16596. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Poźniak, S.; Wojciechowski, T.; Chlubny, L.; Olszyna, A.; Ziemkowska, W.; Jastrzębska, A.M. Influence of modification of Ti 3 C 2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants. RSC Adv. 2019, 9, 4092–4105. [Google Scholar] [CrossRef] [Green Version]
- Rozmysłowska-Wojciechowska, A.; Mitrzak, J.; Szuplewska, A.; Chudy, M.; Woźniak, J.; Petrus, M.; Wojciechowski, T.; Vasilchenko, A.S.; Jastrzębska, A.M. Engineering of 2D Ti3C2 MXene Surface Charge and its Influence on Biological Properties. Materials 2020, 13, 2347. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The-antimicrobial-activity-of-nanoparticles--present-situati. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Karwowska, E. Antibacterial potential of nanocomposite-based materials—A short review. Nanotechnol. Rev. 2017, 6, 243–254. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mohammed Sadiq, I.; Prathna, T.C.; Chandrasekaran, N. Antimicrobial activity of aluminium oxide nanoparticles for potential clinical applications. Sci. against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 245–251. [Google Scholar]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Interaction of Al2O3 nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 2014, 116, 772–783. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Pal, R.; Cameotra, S.S. Antibacterial potential of Al2O3 nanoparticles against multidrug resistance strains of Staphylococcus aureus isolated from skin exudates. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Sadras, S.R. Green synthesized nano silver: Synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J. Colloid Interface Sci. 2017, 499, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Salomoni, R.; Léo, P.; Montemor, A.F.; Rinaldi, B.G.; Rodrigues, M.F.A. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikumar, P.S. Antibacterial Activity of Copper Nanoparticles and Copper Nanocomposites against Escherichia Coli Bacteria. Int. J. Sci. 2016, 2, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Zia, R.; Riaz, M.; Farooq, N.; Qamar, A.; Anjum, S. Antibacterial activity of Ag and Cu nanoparticles synthesized by chemical reduction method: A comparative analysis. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pallela, P.N.V.K.; Pammi, S.V.N.; Padavala, V.S.; Kolapalli, V.R.M. Synergetic antibacterial and anticarcinogenic effects of Annona squamosa leaf extract mediated silver nano particles. Mater. Sci. Semicond. Process. 2019, 100, 301–309. [Google Scholar] [CrossRef]
- Anuj, S.A.; Gajera, H.P.; Hirpara, D.G.; Golakiya, B.A. Bactericidal assessment of nano-silver on emerging and re-emerging human pathogens. J. Trace Elem. Med. Biol. 2019, 51, 219–225. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef]
- Ulloa-Ogaz, A.L.; Piñón-Castillo, H.A.; Muñoz-Castellanos, L.N.; Athie-García, M.S.; Ballinas-Casarrubias, M.D.L.; Murillo-Ramirez, J.G.; Flores-Ongay, L.Á.; Duran, R.; Orrantia-Borunda, E. Oxidative damage to Pseudomonas aeruginosa ATCC 27833 and Staphylococcus aureus ATCC 24213 induced by CuO-NPs. Environ. Sci. Pollut. Res. 2017, 24, 22048–22060. [Google Scholar] [CrossRef]
- Tontini, G.; Greaves, M.; Ghosh, S.; Bayram, V.; Barg, S. MXene-based 3D porous macrostructures for electrochemical energy storage. J. Phys. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Gabbay, J.; Borkow, G.; Mishal, J.; Magen, E.; Zatcoff, R.; Shemer-Avni, Y. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006, 35, 323–335. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Guibert, G.; Mikhailov, S.; Gedanken, A. Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Damm, C.; Münstedt, H.; Rösch, A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J. Mater. Sci. 2007, 42, 6067–6073. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless melt-electrospinning of polypropylene nanofibres. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Al-Yaseri, I.; Morgan, S.; Retzlaff, W. Assessment of Current Models Ability to Describe Chlorine Decay and Appraisal of Water Spectroscopic Data as Model Inputs. J. Environ. Eng. 2013, 139, 1152–1161. [Google Scholar] [CrossRef]
- Rahman, K.U.; Ferreira-Neto, E.P.; Rahman, G.U.; Parveen, R.; Monteiro, A.S.; Rahman, G.; Van Le, Q.; Domeneguetti, R.R.; Ribeiro, S.J.L.; Ullah, S. Flexible bacterial cellulose-based BC-SiO2-TiO2-Ag membranes with self-cleaning, photocatalytic, antibacterial and UV-shielding properties as a potential multifunctional material for combating infections and environmental applications. J. Environ. Chem. Eng. 2020, 104708. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, J.; Zhu, J.; Peng, D.; Zhang, Y.; Zhang, Y. Self-cleaning, antibacterial mixed matrix membranes enabled by photocatalyst Ti-MOFs for efficient dye removal. J. Memb. Sci. 2020, 610, 118219. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Li, R.; Ren, Y.; Zhao, P.; Wang, J.; Liu, J.; Zhang, Y. Graphitic carbon nitride (g-C3N4) nanosheets functionalized composite membrane with self-cleaning and antibacterial performance. J. Hazard. Mater. 2019, 365, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tian, Z.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Self-cleaning and flexible filters based on aminopyridine conjugated microporous polymers nanotubes for bacteria sterilization and efficient PM2.5 capture. Sci. Total Environ. 2020, 142594. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Popa, M.; Chifiriuc, M.C.; Iordache, O.; Dumitrescu, I.; Diamandescu, L.; Dinischiotu, A. Reduced graphene oxide/TiO2 nanocomposites coating of cotton fabrics with antibacterial and self-cleaning properties. J. Ind. Text. 2019, 49, 277–293. [Google Scholar] [CrossRef]
- Choi, H.; Antoniou, M.G.; de la Cruz, A.A.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrzębska, A.; Karwowska, E.; Olszyna, A. Influence of the Staphylococcus aureus Bacteria cells on the Zeta Potential of Graphene Oxide Modified with Alumina Nanoparticles in Electrolyte and Drinking Water Environment. In Proceedings of the 2nd International Congress on Energy Efficiency and Energy Related Materials (ENEFM2014), Oludeniz, Fethiye/Mugla, Turkey, 16–19 October 2014; pp. 245–250. [Google Scholar] [CrossRef]
- Jastrzebska, A.; Karwowska, E.; Basiak, D.; Zawada, A.; Ziemkowska, W.; Wojciechowski, T.; Jakubowska, D.; Olszyna, A. Biological activity and bio-sorption properties of the Ti2C studied by means of zeta potential and SEM. Int. J. Electrochem. Sci. 2017, 12, 2159–2172. [Google Scholar] [CrossRef] [Green Version]
- Sankar, R.; Rahman, P.K.S.M.; Varunkumar, K.; Anusha, C.; Kalaiarasi, A.; Shivashangari, K.S.; Ravikumar, V. Facile synthesis of Curcuma longa tuber powder engineered metal nanoparticles for bioimaging applications. J. Mol. Struct. 2017, 1129, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Teixeira, J.A.; Addali, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Salih, H.H.M.; El Badawy, A.M.; Tolaymat, T.M.; Patterson, C.L. Removal of Stabilized Silver Nanoparticles from Surface Water by Conventional Treatment Processes. Adv. Nanoparticles 2019, 08, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, J.; Ebel, N.; Schlücker, E.; Leipertz, A. Characterization of Escherichia coli suspensions using UV/Vis/NIR absorption spectroscopy. Anal. Methods 2010, 2, 123–128. [Google Scholar] [CrossRef]








| Ti3C2/Al2O3/Ag/Cu | Al2O3/Ag/Cu | |||||
|---|---|---|---|---|---|---|
| Content of Metallic Nanoparticles | 2 wt % | 4 wt % | 8 wt % | 2 wt % | 4 wt % | 8 wt % |
| C9H21O3Al [mg] | 20 | 20 | 20 | 378 | 356 | 312 |
| C2H3AgO2 [mg] | 10 | 20 | 40 | 10 | 20 | 40 |
| C4H6O4Cu [mg] | 12 | 24 | 48 | 12 | 24 | 48 |
| Ti3C2 [mg] | 378 | 356 | 312 | – | – | – |
| Bacteria | The Growth Inhibition Zones (mm) for Different Noble Metal Contents in Ti3C2/Al2O3/Ag/Cu Nanocomposite | ||
|---|---|---|---|
| 2 wt % | 4 wt % | 8 wt % | |
| Escherichia coli | 0.70 ± 0.10 | 1.31 ± 0.18 | 2.40 ± 0.17 |
| Pseudomonas putida | 1.70 ± 0.10 | 1.93 ± 0.16 | 4.47 ± 0.25 |
| Sarcina lutea | 1.50 ± 0.14 | 0.29 ± 0.08 | 0.60 ± 0.06 |
| Staphylococcus aureus | 2.02 ± 0.08 | 1.08 ± 0.10 | 2.42 ± 0.09 |
| Bacillus subtilis | 0.69 ± 0.09 | 0.27 ± 0.03 | 2.43 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczak, M.; Karwowska, E.; Rozmysłowska-Wojciechowska, A.; Petrus, M.; Woźniak, J.; Mitrzak, J.; Jastrzębska, A.M. Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment. Materials 2021, 14, 182. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14010182
Jakubczak M, Karwowska E, Rozmysłowska-Wojciechowska A, Petrus M, Woźniak J, Mitrzak J, Jastrzębska AM. Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment. Materials. 2021; 14(1):182. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14010182
Chicago/Turabian StyleJakubczak, Michał, Ewa Karwowska, Anita Rozmysłowska-Wojciechowska, Mateusz Petrus, Jarosław Woźniak, Joanna Mitrzak, and Agnieszka M. Jastrzębska. 2021. "Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment" Materials 14, no. 1: 182. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14010182