Structure, Luminescence, and Magnetic Properties of Crystalline Manganese Tungstate Doped with Rare Earth Ion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MnWO4 and Rare Earth Doped with MnWO4
2.2. Chraraterization
2.3. Fabrication of MnWO4:Dy3+ Epoxy Composite
3. Results and Discussion
3.1. Crystallinity of MnWO4 According to Various Sintering Temperatures
3.2. Crystallinity, and Magnetic and Chemical State of MnWO4 Doped with Dy3+ Ions
3.3. Luminescence and Morphology Properties of MnWO4 Doped with Dy3+ Ions
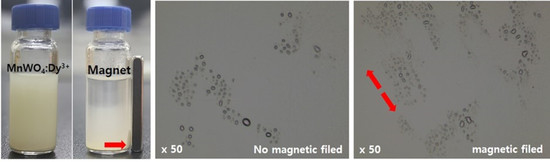
3.4. MnWO4:Dy3+ Particle Aligned in Epoxy Composite by Magnetic Field
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downing, E.; Hesselink, L.; Ralston, J.; Macfarlane, R. A Three-Color, Solid-State, Three-Dimensional Display. Science 1996, 273, 1185–1189. [Google Scholar] [CrossRef]
- Cooper, T.; De Leeuw, N. A combined ab initio and atomistic simulation study of the surface and interfacial structures and energies of hydrated scheelite: Introducing a CaWO4 potential model. Surf. Sci. 2003, 531, 159–176. [Google Scholar] [CrossRef]
- Nivetha, P.; Kavitha, B.; Kalanithi, M. Investigation of photocatalytic and antimicrobial activities of BaWO4–MoS2 nanoflowers. J. Sci. Adv. Mater. Devices 2021, 6, 65–74. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J. Local atomic and electronic structure of tungsten ions in AWO4 crystals of scheelite and wolframite types. Radiat. Meas. 2001, 33, 583–586. [Google Scholar] [CrossRef]
- Koepke’, C.; Wojtowicz, A.J.; Lempicki, A. Luminescence Excited-state absorption in excimer-pumped CaWO4 crystals. J. Lumin. 1993, 54, 345–355. [Google Scholar] [CrossRef]
- Grobelna, B.; Lipowska, B.; Kłonkowski, A.M. Energy transfer in calcium tungstate doped with Eu(III) or Tb(III) ions incorporated into silica xerogel. J. Alloys Compd. 2006, 419, 191–196. [Google Scholar] [CrossRef]
- Naik, S.; Salker, A. Solid state studies on cobalt and copper tungstates nano materials. Solid State Sci. 2010, 12, 2065–2072. [Google Scholar] [CrossRef]
- Rajagopal, S.; Nataraj, D.; Mangalaraj, D.; Djaoued, Y.; Robichaud, J.; Khyzhun, O.Y. Controlled Growth of WO3 Nanostructures with Three Different Morphologies and Their Structural, Optical, and Photodecomposition Studies. Nanoscale Res. Lett. 2009, 4, 1335–1342. [Google Scholar] [CrossRef] [Green Version]
- López, X.A.; Fuentes, A.F.; Zaragoza, M.M.; Guillén, J.A.D.; Gutiérrez, J.S.; Ortiz, A.L.; Collins-Martínez, V. Synthesis, characterization and photocatalytic evaluation of MWO4 (M = Ni, Co, Cu and Mn) tungstates. Int. J. Hydrogen Energy 2016, 41, 23312–23317. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Huo, J.; Wang, W. A simple synthesis of MnWO4 nanoparticles as a novel energy storage material. Mater. Chem. Phys. 2015, 167, 22–27. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Kim, J.; Shim, Y.-S.; Hwang, D.; Son, C. Structure and Photoluminescence Properties of Rare-Earth (Dy3+, Tb3+, Sm3+)-Doped BaWO4 Phosphors Synthesized via Co-Precipitation for Anti-Counterfeiting. Materials 2020, 13, 4165. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, X.; Li, X.; He, J.; Wang, B. Synthesis and Luminescence of BaWO4:Ln3+ (Ln = Eu, Tb, and Dy) Powders. J. Electron. Mater. 2014, 43, 3534–3538. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Xue, Z.; Zhou, J.; Li, J.; Hong, J.; You, X. Morphology control of MnWO4 nanocrystals by a solvothermal route. J. Mat. Chem. 2003, 13, 1132–1135. [Google Scholar] [CrossRef]
- Shen, Y.-J.; Zhang, Y.; Gao, F.; Yang, G.-S.; Lai, X.-P. Influence of Temperature on the Microstructure Deterioration of Sandstone. Energies 2018, 11, 1753. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.N.; Hussain, T.; Khan, M.A.; Ahmad, M. Structural, magnetic, dielectric and high frequency response of synthesized rare earth doped bismuth nano garnets (BIG). Results Phys. 2018, 10, 784–793. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirai, H.; Matsuoka, T.; Ohishi, Y.; Yagi, T.; Ohtake, M.; Yamamoto, Y.; Nakano, S.; Irifune, T. Phase changes of filled ice Ih methane hydrate under low temperature and high pressure. J. Chem. Phys. 2013, 139, 104701. [Google Scholar] [CrossRef]
- Dai, R.; Ding, X.; Wang, Z.; Zhang, Z. Pressure and temperature dependence of Raman scattering of MnWO4. Chem. Phys. Lett. 2013, 586, 76–80. [Google Scholar] [CrossRef]
- Iliev, M.; Gospodinov, M.M.; Litvinchuk, A.P. Raman spectroscopy of MnWO4. Phys. Rev. B 2009, 80, 212302. [Google Scholar] [CrossRef]
- Maczka, M.; Ptak, M.; Da Silva, K.P.; Freire, P.D.T.C.; Hanuza, J. High-pressure Raman scattering and an anharmonicity study of multiferroic wolframite-type Mn0.97Fe0.03WO4. J. Physics Condens. Matter 2012, 24, 345403. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fuertes, J.; Friedrich, A.; Gomis, O.; Errandonea, D.; Morgenroth, W.; Sans, J.A.; Santamaria-Perez, D. High-pressure structural phase transition inMnWO4. Phys. Rev. B 2015, 91, 104109. [Google Scholar] [CrossRef] [Green Version]
- Macavei, J.; Schulz, H. The crystal structure of wolframite type tungstates at high pressure. Z. Krist. Cryst. Mater. 1993, 207, 193–208. [Google Scholar] [CrossRef]
- Iliev, M.N.; Guo, H.; Gupta, A. Raman spectroscopy evidence of strong spin-phonon coupling in epitaxial thin films of the double perovskite La2NiMnO6. Appl. Phys. Lett. 2007, 90, 151914. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Long, M.-Q.; Wang, Y.-P. Paramagnetic phases of two-dimensional magnetic materials. Phys. Rev. B 2020, 102, 214417. [Google Scholar] [CrossRef]
- Jiang, F.; Pang, Z.; Yuan, H.; Wei, Z.; Xie, W.; Wu, Z.; Han, S. Room temperature ferromagnetic properties of dysprosium-doped tris(8-hydroxyquinoline) aluminum: Experimental and theoretical investigation. RSC Adv. 2016, 6, 43780–43785. [Google Scholar] [CrossRef]
- Muthamizh, S.; Suresh, R.; Giribabu, K.; Manigandan, R.; Kumar, S.P.; Munusamy, S.; Narayanan, V. MnWO4 nanocapsules: Synthesis, characterization and its electrochemical sensing property. J. Alloys Compd. 2015, 619, 601–609. [Google Scholar] [CrossRef]
- Dong, F.; Sattayasamitsathit, S.; Zhang, Y.X.; Zhou, Y. Materials Chemistry for Sustainability and Energy. J. Chem. 2014, 2014, 1–3. [Google Scholar] [CrossRef]
- Gokhale, S.; Ahmed, N.; Mahamuni, S.; Rao, V.; Nigavekar, A.; Kulkarni, S. XPS and XRD investigations of Dy/Si interface. Surf. Sci. 1989, 210, 85–98. [Google Scholar] [CrossRef]
- Rydberg, S.; Engholm, M.; Rydberg, S.; Engholm, M. Charge transfer processes and ultraviolet induced absorption in Yb:YAG single crystal laser materials Charge transfer processes and ultraviolet induced absorption in Yb:YAG single crystal laser ma-terials. J. Appl. Phys. 2013, 11, 223510. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Brahme, N.; Bisen, D.P.; Dewangan, P. Cool white light emission from Dy3+ activated alkaline alumino silicate phosphors. Opt. Express 2018, 26, 29495–29508. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Xia, Z.; Du, H. Synthesis and luminescence properties of novel LiSrPO4:Dy3+ phosphor. Mater. Res. Bull. 2011, 46, 2179–2182. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-Y.; Yi, S.-S.; Hwang, D.-H.; Son, C.-S. Structure, Luminescence, and Magnetic Properties of Crystalline Manganese Tungstate Doped with Rare Earth Ion. Materials 2021, 14, 3717. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14133717
Jung J-Y, Yi S-S, Hwang D-H, Son C-S. Structure, Luminescence, and Magnetic Properties of Crystalline Manganese Tungstate Doped with Rare Earth Ion. Materials. 2021; 14(13):3717. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14133717
Chicago/Turabian StyleJung, Jae-Young, Soung-Soo Yi, Dong-Hyun Hwang, and Chang-Sik Son. 2021. "Structure, Luminescence, and Magnetic Properties of Crystalline Manganese Tungstate Doped with Rare Earth Ion" Materials 14, no. 13: 3717. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14133717