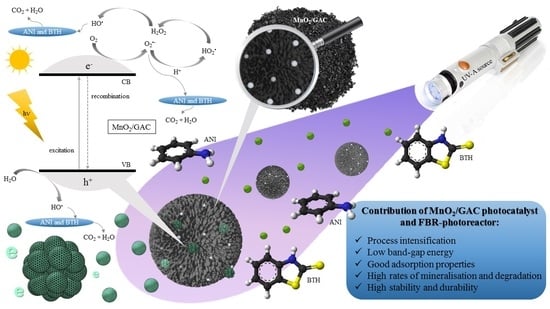
Removal of Aniline and Benzothiazole Wastewaters Using an Efficient MnO2/GAC Catalyst in a Photocatalytic Fluidised Bed Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalyst Preparation
2.3. Analyses
2.3.1. Chemical Analysis
2.3.2. Catalytic Material Characterisation
2.4. UV-A Pilot Plant Description and Experimental Procedure
3. Results and Discussion
3.1. Catalyst Characterisation
3.2. Reaction in the FBR-Photoreactor
3.2.1. FBR Sizing
3.2.2. Catalytic Material and Surface Velocity of Fluid in the FBR
3.3. MnO2/GAC Composite Testing in Photocatalysis for Aniline and Benzothiazole Removal from Wastewater
3.3.1. Kinetics of ANI and BTH Adsorption and Photodegradation with the MnO2/GAC-3 Composite
3.3.2. Effects of pH and Catalyst Dose during the Photocatalysis Process for ANI and BTH Removal with the Composite MnO2/GAC-3
3.4. Stability of MnO2/GAC-3 after Photocatalysis and Treatment Cost Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bello, M.M.; Abdul Raman, A.A.; Purushothaman, M. Applications of Fluidized Bed Reactors in Wastewater Treatment—A Review of the Major Design and Operational Parameters. J. Clean. Prod. 2017, 141, 1492–1514. [Google Scholar] [CrossRef]
- Aitken, D.; Rivera, D.; Godoy-Faúndez, A.; Holzapfel, E. Water Scarcity and the Impact of the Mining and Agricultural Sectors in Chile. Sustainability 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic pH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; de Luis, A.; Lombrana, J.I. Analysis of the Effect of the Operational Variants in a Combined Adsorption-Ozonation Process with Granular Activated Carbon for the Treatment of Phenol Wastewater. React. Chem. Eng. 2020, 5, 760–778. [Google Scholar] [CrossRef]
- Tisa, F.; Abdul Raman, A.A.; Wan Daud, W.M.A. Applicability of Fluidized Bed Reactor in Recalcitrant Compound Degradation through Advanced Oxidation Processes: A Review. J. Environ. Manag. 2014, 146, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J. An Efficient Catalytic Process for the Treatment of Genotoxic Aniline Wastewater Using a New Granular Activated Carbon-Supported Titanium Dioxide Composite. J. Clean. Prod. 2019, 228, 1282–1295. [Google Scholar] [CrossRef]
- Hering, J.G.; Maag, S.; Schnoor, J.L. A Call for Synthesis of Water Research to Achieve the Sustainable Development Goals by 2030. Environ. Sci. Technol. 2016, 50, 6122–6123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations About the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 7 February 2021).
- Valdés, H.; Zaror, C.A.; Jekel, M. Removal of Benzothiazole from Contaminated Waters by Ozonation: The Role of Direct and Indirect Ozone Reactions. J. Adv. Oxid. Technol. 2016, 19, 338–346. [Google Scholar] [CrossRef]
- Corsi, S.R.; Zitomer, D.H.; Field, J.A.; Cancilla, D.A. Nonylphenol Ethoxylates and Other Additives in Aircraft Deicers, Antiicers, and Waters Receiving Airport Runoff. Environ. Sci. Technol. 2003, 37, 4031–4037. [Google Scholar] [CrossRef]
- Seitz, W.; Winzenbacher, R. A Survey on Trace Organic Chemicals in a German Water Protection Area and the Proposal of Relevant Indicators for Anthropogenic Influences. Environ. Monit. Assess. 2017, 189, 244. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An Overview on the Advanced Oxidation Processes Applied for the Treatment of Water Pollutants Defined in the Recently Launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- De Wever, H.; Verachtert, H. Biodegradation and Toxicity of Benzothiazoles. Water Res. 1997, 31, 2673–2684. [Google Scholar] [CrossRef]
- Felis, E.; Sochacki, A.; Magiera, S. Degradation of Benzotriazole and Benzothiazole in Treatment Wetlands and by Artificial Sunlight. Water Res. 2016, 104, 441–448. [Google Scholar] [CrossRef]
- Mei-yan, X.; Can, D.; Godefroid, B.; Jian, Y. Treatment of Pharmaceutical Wastewater Containing Recalcitrant Compounds in a Fenton-Coagulation Process. J. Environ. Sci. 2006, 18, 459–463. [Google Scholar]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Adsorptive Removal of Aniline by Granular Activated Carbon from Aqueous Solutions with Catechol and Resorcinol. Environ. Technol. 2012, 33, 773–781. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Hu, P.; Huang, R. Removal of Aniline from Aqueous Solutions by Activated Carbon Coated by Chitosan. J. Water Reuse Desalin. 2015, 5, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Sumegova, L.; Derco, J.; Melicher, M. Degradation of Benzothiazole by Ozonation and Adsorptive Ozonation. Chem. Biochem. Eng. Q. 2015, 29, 63–66. [Google Scholar] [CrossRef]
- Jing, Z.; Cao, S.; Yu, T.; Hu, J. Degradation Characteristics of Aniline with Ozonation and Subsequent Treatment Analysis. J. Chem. 2015, 2015, 905921. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J. Heterogeneous Catalytic Ozonation of Aniline-Contaminated Waters: A Three-Phase Modelling Approach Using TiO2/GAC. Water 2020, 12, 3448. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J.; Zúñiga, V.; Rituerto, J.M. Analysis of a Hybrid Suspended-Supported Photocatalytic Reactor for the Treatment of Wastewater Containing Benzothiazole and Aniline. Water 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Canle, L.M.; Santaballa, J.A.; Vulliet, E. On the Mechanism of TiO2-Photocatalyzed Degradation of Aniline Derivatives. J. Photochem. Photobiol. A Chem. 2005, 175, 192–200. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent Advances in Photocatalytic Treatment of Pollutants in Aqueous Media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.T.; Machado, B.F.; Ribeiro, A.; Moreira, I.; Rosário, M.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L. Catalytic Properties of Carbon Materials for Wet Oxidation of Aniline. J. Hazard. Mater. 2008, 159, 420–426. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, Y.; Cao, H.; Guo, Z.; Wen, J.; Shi, Y. Enhanced Removal of Benzothiazole in Persulfate Promoted Wet Air Oxidation via Degradation and Synchronous Polymerization. Chem. Eng. J. 2019, 370, 208–217. [Google Scholar] [CrossRef]
- Ferreiro, C.; Gómez-Motos, I.; Lombraña, J.I.; de Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of Emerging Concern Removal in an Effluent of Wastewater Treatment Plant under Biological and Continuous Mode Ultrafiltration Treatment. Sustainability 2020, 12, 725. [Google Scholar] [CrossRef] [Green Version]
- Rahmat, M.; Rehman, A.; Rahmat, S.; Bhatti, H.N.; Iqbal, M.; Khan, W.S.; Bajwa, S.Z.; Rahmat, R.; Nazir, A. Highly Efficient Removal of Crystal Violet Dye from Water by MnO2 Based Nanofibrous Mesh/Photocatalytic Process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Shivaraju, H.P.; Midhun, G.; Anil Kumar, K.M.; Pallavi, S.; Pallavi, N.; Behzad, S. Degradation of Selected Industrial Dyes Using Mg-Doped TiO2 Polyscales under Natural Sun Light as an Alternative Driving Energy. Appl. Water Sci. 2017, 7, 3937–3948. [Google Scholar] [CrossRef] [Green Version]
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent Developments in MnO2-Based Photocatalysts for Organic Dye Removal: A Review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778. [Google Scholar] [CrossRef]
- Orha, C.; Pode, R.; Manea, F.; Lazau, C.; Bandas, C. Titanium Dioxide-Modified Activated Carbon for Advanced Drinking Water Treatment. Process. Saf. Environ. Prot. 2017, 108, 26–33. [Google Scholar] [CrossRef]
- Ameta, S. Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press: San Diego, CA, USA, 2018; ISBN 978-0-12-810499-6. [Google Scholar]
- Gomez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Review and Perspectives on the Use of Magnetic Nanophotocatalysts (MNPCs) in Water Treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Munir, S.; Dionysiou, D.D.; Khan, S.B.; Shah, S.M.; Adhikari, B.; Shah, A. Development of Photocatalysts for Selective and Efficient Organic Transformations. J. Photochem. Photobiol. B Biol. 2015, 148, 209–222. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. TiO2 Structures Doped with Noble Metals and/or Graphene Oxide to Improve the Photocatalytic Degradation of Dichloroacetic Acid. Environ. Sci. Pollut. Res. Int. 2017, 24, 12628–12637. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Rivero, M.J.; Ortiz, I. Kinetics of Dodecylbenzenesulphonate Mineralisation by TiO2 Photocatalysis. Appl. Catal. B Environ. 2011, 101, 515–521. [Google Scholar] [CrossRef]
- Bodzek, M.; Rajca, M. Photocatalysis in the Treatment and Disinfection of Water. Part I. Theoretical Backgrounds. Ecol. Chem. Eng. 2012, 19, 489–512. [Google Scholar] [CrossRef]
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.A.; Mangalaraja, R.V.; O’Shea, K.; Dionysiou, D.D. Industrial Synthesis and Characterization of Nanophotocatalysts Materials: Titania. Nanotechnol. Rev. 2016, 5, 467–479. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, Y.; Guo, Y.; Rhodes, G.; Yeom, J.; Li, H.; Zhang, W. Photocatalytic Degradation of Cephalexin by ZnO Nanowires under Simulated Sunlight: Kinetics, Influencing Factors, and Mechanisms. Environ. Int. 2019, 132, 105105. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Wang, X.; Huang, H.; Peng, G.; Wang, S.; Fan, Z. A Novel Photocatalytic Material for Removing Microcystin-LR under Visible Light Irradiation: Degradation Characteristics and Mechanisms. PLoS ONE 2014, 9, e95798. [Google Scholar] [CrossRef] [PubMed]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sui, M.-H.; Chen, Z.-L.; Wang, L.-N. Degradation of Refractory Organic Pollutants by Catalytic Ozonation—Activated Carbon and Mn-Loaded Activated Carbon as Catalysts. Ozone Sci. Eng. 2004, 26, 3–10. [Google Scholar] [CrossRef]
- Tang, N.; Tian, X.; Yang, C.; Pi, Z.; Han, Q. Facile Synthesis of α-MnO2 Nanorods for High-Performance Alkaline Batteries. J. Phys. Chem. Solids 2010, 71, 258–262. [Google Scholar] [CrossRef]
- Lin, H.; Chen, D.; Liu, H.; Zou, X.; Chen, T. Effect of MnO2 Crystalline Structure on the Catalytic Oxidation of Formaldehyde. Aerosol Air Qual. Res. 2017, 17, 1011–1020. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, F.; Cao, H.; Xie, Y.; Xiao, J.; Chen, Y.; Ghazi, Z.A. Selection of Active Phase of MnO2 for Catalytic Ozonation of 4-Nitrophenol. Chemosphere 2017, 168, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Bhattacharyya, K.G. Oxidation of Rhodamine B in Aqueous Medium in Ambient Conditions with Raw and Acid-Activated MnO2, NiO, ZnO as Catalysts. J. Mol. Catal. A Chem. 2014, 391, 121–129. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Camargo, P.H.C.; Wang, J. Investigating the Effect of MnO2 Band Gap in Hybrid MnO2–Au Materials over the SPR-Mediated Activities under Visible Light. J. Mater. Chem. A 2019, 7, 925–931. [Google Scholar] [CrossRef]
- Warsi, M.F.; Bilal, M.; Zulfiqar, S.; Khalid, M.U.; Agboola, P.O.; Shakir, I. Enhanced Visible Light Driven Photocatalytic Activity of MnO2 Nanomaterials and Their Hybrid Structure with Carbon Nanotubes. Mater. Res. Express 2020, 7, 105015. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Yang, X.; Shao, E.; Deng, X.; Liu, N.; Wu, M. Facile Synthesis of Three-Dimensional Mn3O4 Hierarchical Microstructures and Their Application in the Degradation of Methylene Blue. J. Mater. Chem. A 2015, 3, 2934–2941. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, M.; Zhang, Y.; Zhu, C.; Fang, Y.; Li, Y.; Yu, L. 3D Hierarchical Structures MnO2/C: A Highly Efficient Catalyst for Purification of Volatile Organic Compounds with Visible Light Irradiation. Appl. Surf. Sci. 2018, 447, 191–199. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Tsai, M.-T.; Huang, Y.-H. Mineralization and Defluoridation of 2,2,3,3-Tetrafluoro-1-Propanol (TFP) by UV Oxidation in a Novel Three-Phase Fluidized Bed Reactor (3P-FBR). Water Res. 2013, 47, 2325–2330. [Google Scholar] [CrossRef]
- Tian, S.H.; Tu, Y.T.; Chen, D.S.; Chen, X.; Xiong, Y. Degradation of Acid Orange II at Neutral pH Using Fe2(MoO4)3 as a Heterogeneous Fenton-like Catalyst. Chem. Eng. J. 2011, 169, 31–37. [Google Scholar] [CrossRef]
- Moussavi, G.; Aghapour, A.A.; Yaghmaeian, K. The Degradation and Mineralization of Catechol Using Ozonation Catalyzed with MgO/GAC Composite in a Fluidized Bed Reactor. Chem. Eng. J. 2014, 249, 302–310. [Google Scholar] [CrossRef]
- Kanki, T.; Hamasaki, S.; Sano, N.; Toyoda, A.; Hirano, K. Water Purification in a Fluidized Bed Photocatalytic Reactor Using TiO2-Coated Ceramic Particles. Chem. Eng. J. 2005, 108, 155–160. [Google Scholar] [CrossRef]
- Huang, C.-P.; Huang, Y.-H. Application of an Active Immobilized Iron Oxide with Catalytic H2O2 for the Mineralization of Phenol in a Batch Photo-Fluidized Bed Reactor. Appl. Catal. A Gen. 2009, 357, 135–141. [Google Scholar] [CrossRef]
- Al Mgheer, T.; Abdulrazzak, F.H. Oxidation of Multi-Walled Carbon Nanotubes in Acidic and Basic Piranha Mixture. Front. Nanosci. Nanotechnol. 2016, 2, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.H.; Liu, F.; Tan, W.F.; Liu, X.W. Synthesis of Birnessite from the Oxidation of Mn2+ by O2 in Alkali Medium: Effects of Synthesis Conditions. Clays Clay Miner. 2004, 52, 240–250. [Google Scholar] [CrossRef]
- Mijangos, F.; Varona, F.; Villota, N. Changes in Solution Color During Phenol Oxidation by Fenton Reagent. Environ. Sci. Technol. 2006, 40, 5538–5543. [Google Scholar] [CrossRef] [PubMed]
- Morrow, W.; McLean, L. Self-Cleaning UV Reflective Coating. U.S. Patent US20030059549A1, 30 October 2003. [Google Scholar]
- Alhamed, Y.A. Adsorption Kinetics and Performance of Packed Bed Adsorber for Phenol Removal Using Activated Carbon from Dates’ Stones. J. Hazard. Mater. 2009, 170, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Sadiq, M.; Khan, I.; Saeed, K. Manganese Dioxide Nanoparticles/Activated Carbon Composite as Efficient UV and Visible-Light Photocatalyst. Environ. Sci. Pollut. Res. 2019, 26, 5140–5154. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.S.; Radha, K.V. Gas–Liquid Mass Transfer Studies in Inverse Fluidized Bed Biofilm Reactor for the Biodegradation of Industrial Effluent Rich in Phenolic Compounds. Environ. Prog. Sustain. Energy 2016, 35, 433–438. [Google Scholar] [CrossRef]
- Fernández, N.; Montalvo, S.; Borja, R.; Guerrero, L.; Sánchez, E.; Cortés, I.; Colmenarejo, M.F.; Travieso, L.; Raposo, F. Performance Evaluation of an Anaerobic Fluidized Bed Reactor with Natural Zeolite as Support Material When Treating High-Strength Distillery Wastewater. Renew. Energy 2008, 33, 2458–2466. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous Activated Carbon from Industrial Laundry Sewage Sludge: Adsorption Studies of Reactive Dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Papirer, E.; Li, S.; Donnet, J.-B. Contribution to the Study of Basic Surface Groups on Carbons. Carbon 1987, 25, 243–247. [Google Scholar] [CrossRef]
- Ghasemi, B.; Anvaripour, B.; Jorfi, S.; Jaafarzadeh, N. Enhanced Photocatalytic Degradation and Mineralization of Furfural Using UVC/TiO2/GAC Composite in Aqueous Solution. Int. J. Photoenergy 2016, 2016, 2782607. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Hsi, H.-C.; Li, K.-C.; Hou, C.-H. Electrodeposited Manganese Dioxide/Activated Carbon Composite As a High-Performance Electrode Material for Capacitive Deionization. ACS Sustain. Chem. Eng. 2016, 4, 4762–4770. [Google Scholar] [CrossRef]
- Choi, J.R.; Lee, J.W.; Yang, G.; Heo, Y.-J.; Park, S.-J. Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors. Catalysts 2020, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, H.; Jin, S. A New Method of Activated Carbon Loading MnO2 to Formaldehyde Degradation. Adv. Mater. Res. 2011, 332–334, 1743–1746. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese Oxides for Lithium Batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Kazeminezhad, I.; Jaafarzadeh, N.; Ahmadi, M.; Ramezani, Z.; Silva Martinez, S. The Visible-Light Photodegradation of Nonylphenol in the Presence of Carbon-Doped TiO2 with Rutile/Anatase Ratio Coated on GAC: Effect of Parameters and Degradation Mechanism. J. Hazard. Mater. 2018, 350, 108–120. [Google Scholar] [CrossRef]
- Shen, X.; Garces, L.-J.; Ding, Y.; Laubernds, K.; Zerger, R.P.; Aindow, M.; Neth, E.J.; Suib, S.L. Behavior of H2 Chemisorption on Ru/TiO2 Surface and Its Application in Evaluation of Ru Particle Sizes Compared with TEM and XRD Analyses. Appl. Catal. A Gen. 2008, 335, 187–195. [Google Scholar] [CrossRef]
- Almeida, Â.; Calisto, V.; Domingues, M.R.M.; Esteves, V.I.; Schneider, R.J.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Comparison of the Toxicological Impacts of Carbamazepine and a Mixture of Its Photodegradation Products in Scrobicularia Plana. J. Hazard. Mater. 2017, 323, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, S.-G.; Fan, M.; Gong, W.-X.; Gao, B.-Y. Characteristics and Defluoridation Performance of Granular Activated Carbons Coated with Manganese Oxides. J. Hazard. Mater. 2009, 168, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, N.; Feng, C.; Liu, Y.; Ma, N.; Deng, J.; Xing, L.; Gao, Y. Ozonation Catalyzed by Iron- and/or Manganese-Supported Granular Activated Carbons for the Treatment of Phenol. Environ. Sci. Pollut. Res. Int. 2019, 26, 21022–21033. [Google Scholar] [CrossRef] [PubMed]
- Rubinson, K.A.; Rubinson, J.F. Contemporary Instrumental Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2000; ISBN 978-0-13-790726-7. [Google Scholar]
- Hu, E.; Wu, X.; Shang, S.; Tao, X.; Jiang, S.; Gan, L. Catalytic Ozonation of Simulated Textile Dyeing Wastewater Using Mesoporous Carbon Aerogel Supported Copper Oxide Catalyst. J. Clean. Prod. 2016, 112, 4710–4718. [Google Scholar] [CrossRef]
- Spivey, J.J.; Agarwal, S.K.; Bartholemew, C.H. H2 adsorption on supported noble metals and its use in determining metal dispersion. Catalysis 1994, 11, 93–126. [Google Scholar] [CrossRef]
- Askaripour, H.; Molaei Dehkordi, A. Effects of Initial Static Bed Height on Fractional Conversion and Bed Pressure Drop in Tapered-in and Tapered-out Fluidized Bed Reactors. Int. J. Multiph. Flow 2016, 79, 50–61. [Google Scholar] [CrossRef]
- Ochieng, A.; Odiyo, J.O.; Mutsago, M. Biological Treatment of Mixed Industrial Wastewaters in a Fluidised Bed Reactor. J. Hazard. Mater. 2003, 96, 79–90. [Google Scholar] [CrossRef]
- Nam, W.; Woo, K.; Han, G. Photooxidation of Anionic Surfactant (Sodium Lauryl Sulfate) in a Three-Phase Fluidized Bed Reactor Using TiO2/SiO2 Photocatalyst. J. Ind. Eng. Chem. 2009, 15, 348–353. [Google Scholar] [CrossRef]
- Shim, S.; Won, S.; Reza, A.; Kim, S.; Ahmed, N.; Ra, C. Design and Optimization of Fluidized Bed Reactor Operating Conditions for Struvite Recovery Process from Swine Wastewater. Processes 2020, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Zarekar, S.; Bück, A.; Jacob, M.; Tsotsas, E. Numerical Study of the Hydrodynamics of Fluidized Beds Operated under Sub-Atmospheric Pressure. Chem. Eng. J. 2019, 372, 1134–1153. [Google Scholar] [CrossRef]
- Delebarre, A.; Morales, J.-M.; Ramos, L. Influence of the Bed Mass on Its Fluidization Characteristics. Chem. Eng. J. 2004, 98, 81–88. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol Adsorption on High Microporous Activated Carbons Prepared from Oily Sludge: Equilibrium, Kinetic and Thermodynamic Studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef] [PubMed]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The Effect of Doping Elements on Photocatalytic Activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Zhou, L.; Zhang, L. Bi2WO6 Nanocrystals with High Photocatalytic Activities under Visible Light. J. Phys. Chem. C 2008, 112, 10407–10411. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Kinetic Modelling for the Photocatalytic Degradation of Phenol by Using TiO2-Coated Glass Raschig Rings under Simulated Solar Light. J. Chem. Technol. Biotechnol. 2016, 91, 346–352. [Google Scholar] [CrossRef]
- Chen, P.H.; Jenq, C.H. Kinetics of Photocatalytic Oxidation of Trace Organic Compounds over Titanium Dioxide. Environ. Int. 1998, 24, 871–879. [Google Scholar] [CrossRef]
- Saroyan, H.; Kyzas, G.Z.; Deliyanni, E.A. Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide. Processes 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- David, A.; Vedhi, C. Synthesis of Nano Co3O4-MnO2-ZrO2 Mixed Oxides for Visible-Light Photocatalytic Activity. Int. J. Adv. Res. Sci. Eng. Technol. 2017, 6, 613–623. [Google Scholar]
- Sánchez, L.; Peral, J.; Domènech, X. Photocatalyzed Destruction of Aniline in UV-Illuminated Aqueous TiO2 Suspensions. Electrochim. Acta 1997, 42, 1877–1882. [Google Scholar] [CrossRef]
- Yang, H.; Bard, A.J. The Application of Fast Scan Cyclic Voltammetry. Mechanistic Study of the Initial Stage of Electropolymerization of Aniline in Aqueous Solutions. J. Electroanal. Chem. 1992, 339, 423–449. [Google Scholar] [CrossRef]
- De Wever, H.; Vereecken, K.; Stolz, A.; Verachtert, H. Initial Transformations in the Biodegradation of Benzothiazoles by Rhodococcus Isolates. Appl. Environ. Microbiol. 1998, 64, 3270–3274. [Google Scholar] [CrossRef] [Green Version]
- Derco, J.; Kassai, A.; Melicher, M.; Dudas, J. Removal of the 2-Mercaptobenotiazole from Model Wastewater by Ozonation. Sci. World J. 2014, 2014, e173010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhang, G.; Zhang, Z. TiO2-Based Catalysts for Photocatalytic Reduction of Aqueous Oxyanions: State-of-the-Art and Future Prospects. Environ. Int. 2020, 136, 105453. [Google Scholar] [CrossRef]
- Ma, J.; Minakata, D.; O’Shea, K.; Bai, L.; Dionysiou, D.D.; Spinney, R.; Xiao, R.; Wei, Z. Determination and Environmental Implications of Aqueous-Phase Rate Constants in Radical Reactions. Water Res. 2021, 190, 116746. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Activated Carbon as Photocatalyst of Reactions in Aqueous Phase. Appl. Catal. B Environ. 2013, 142–143, 694–704. [Google Scholar] [CrossRef]
- Chiam, S.-L.; Yeoh, F.Y.; Pung, S.-Y. Heavy Metal Ions Removal Using Beta-MnO2 Particles under UV Irradiation; Amer Inst Physics: Melville, NY, USA, 2020; ISBN 978-0-7354-2030-4. [Google Scholar]
- Chen, X.; Zhou, J.; Chen, Y.; Zhou, Y.; Ding, L.; Liang, H.; Li, X. Degradation of Tetracycline Hydrochloride by Coupling of Photocatalysis and Peroxymonosulfate Oxidation Processes Using CuO-BiVO4 Heterogeneous Catalyst. Process. Saf. Environ. Prot. 2021, 145, 364–377. [Google Scholar] [CrossRef]
- Hemmati Borji, S.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Javadi, A.H. Investigation of Photocatalytic Degradation of Phenol by Fe(III)-Doped TiO2 and TiO2 Nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Arriaga, F.; Maldonado, M.I.; Gimenez, J.; Esplugas, S.; Malato, S. Abatement of Ibuprofen by Solar Photocatalysis Process: Enhancement and Scale Up. Catal. Today 2009, 144, 112–116. [Google Scholar] [CrossRef]
- Reyes, C.; Fernández, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and Inactivation of Tetracycline by TiO2 Photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Gallay, R.; Pulgarín, C.; Maldonado, M.I.; Malato, S.; Gernjak, W. Comparison of Photo-Fenton Treatment and Coupled Photo-Fenton and Biological Treatment for Detoxification of Pharmaceutical Industry Contaminants. J. Adv. Oxid. Technol. 2008, 11, 261–269. [Google Scholar] [CrossRef]
- Lekshmi, K.P.V.; Yesodharan, S.; Yesodharan, E.P. MnO2 and MnO2/TiO2 mediated, persulphate enhanced photocatalysis for the removal of indigo carmine dye pollutant from water. Eur. Chem. Bull. 2017, 6, 177–191. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, M.F.; Bellato, C.R.; Mounteer, A.H.; Ferreira, S.O.; Milagres, J.L.; Miranda, L.D.L. Enhanced Photocatalytic Activity of TiO2-Impregnated with MgZnAl Mixed Oxides Obtained from Layered Double Hydroxides for Phenol Degradation. Appl. Surf. Sci. 2015, 357, 1765–1775. [Google Scholar] [CrossRef]
- Krichevskaya, M.; Klauson, D.; Portjanskaja, E.; Preis, S. The Cost Evaluation of Advanced Oxidation Processes in Laboratory and Pilot-Scale Experiments. Ozone Sci. Eng. 2011, 33, 211–223. [Google Scholar] [CrossRef]
- European Commission. Eurostat Regional Yearbook 2020. Stat. Books 2020, 1, 1–196. [Google Scholar] [CrossRef]
















| Parameter | Value |
|---|---|
| Aniline (mg L−1) | 12.0 ± 0.5 |
| Benzothiazole (mg L−1) | 12.0 ± 0.6 |
| Dissolved oxygen (mg O2 L−1) | 7.5 ± 0.37 |
| pH | 7.8 ± 0.1 |
| Conductivity at 20 °C (µS cm−1) | 605 ± 30 |
| Chemical oxygen demand (mg O2 L−1) | 45.46 ± 2.27 |
| Total organic carbon (mg C L−1) | 16.73 ± 0.53 |
| Temperature (°C) | 26 ± 0.1 |
| Turbidity (NTU) | 1.01 ± 0.05 |
| Aromatic ring rupture (AU) | 2.453 ± 0.122 |
| Colour (AU) | 0.717 ± 0.03 |
| Nitrite (mg NO2 L−1) | 0.041 ± 0.001 |
| Nitrate (mg NO3 L−1) | 1.6 ± 0.1 |
| Chloride (mg Cl L−1) | 31.7 ± 1.6 |
| Total phosphorus (mg P L−1) | 0.05 ± 0.01 |
| Phosphates (mg PO4 L−1) | 0.07 ± 0.01 |
| Total ammonia (mg NH4 L−1) | 0.14 ± 0.01 |
| Sample | SBET, m2 g−1 | Sext, m2 g−1 | VT, cm3 g−1 | Vμ, cm3 g−1 | VM, cm3 g−1 | VM/VT·100 % | Vμ/VT·100 % | DP, Å |
|---|---|---|---|---|---|---|---|---|
| α-MnO2 | 23.5 | 10.5 | 0.07 | 0.05 | 0.02 | 28.6 | 71.4 | 127.7 |
| Hydrodarco® 3000 GAC | 601.2 | 276.4 | 0.51 | 0.14 | 0.35 | 68.6 | 27.5 | 83.98 |
| MnO2/GAC-1 | 496.1 | 294.1 | 0.49 | 0.09 | 0.38 | 77.6 | 18.0 | 81.28 |
| MnO2/GAC-2 | 556.3 | 305.8 | 0.51 | 0.1 | 0.38 | 74.5 | 19.6 | 80.50 |
| MnO2/GAC-3 | 664.1 | 345.49 | 0.62 | 0.13 | 0.48 | 77.4 | 21.0 | 88.03 |
| Properties | Sample | ||||
|---|---|---|---|---|---|
| α-MnO2 | Hydrodarco® 3000 GAC | MnO2/GAC-1 | MnO2/GAC-2 | MnO2/GAC-3 | |
| pHPZC | 2.60 | 7.25 | 7.10 | 6.85 | 6.61 |
| Disp, % | — | — | 15.5 | 17.3 | 21.0 |
| MnO2, % | N/A | N/A | 1.34 | 2.07 | 3.78 |
| Al2O3, % | — | 0.66 | 0.41 | 0.57 | 0.77 |
| Fe2O3, % | — | 0.26 | 0.15 | 0.36 | 0.34 |
| SiO2, % | — | 6.96 | 7.27 | 7.72 | 6.02 |
| MgO, % | — | 0.15 | 0.23 | 0.06 | 0.23 |
| CaO, % | — | 0.11 | 0.15 | 0.11 | 0.09 |
| Na2O, % | — | 0.01 | 0.01 | 0.01 | 0.01 |
| K2O, % | — | 0.21 | 0.18 | 0.30 | 0.16 |
| TiO2, % | — | 0.17 | 0.14 | 0.38 | 0.10 |
| P2O5, % | — | 0.01 | 0.01 | 0.01 | 0.01 |
| S, % | — | 0.21 | 0.34 | 0.22 | 0.12 |
| Kinetic Model | Parameters | Process | |||
|---|---|---|---|---|---|
| Adsorption | Photocatalysis | ||||
| Aniline | Benzothiazole | Aniline | Benzothiazole | ||
| Pseudo-1st-order | qe (mg g−1) | 18.16 | 14.12 | 1.81 | 1.84 |
| k1 (h−1) | 0.479 | 0.509 | 1.208 | 0.914 | |
| R2 | 0.663 | 0.688 | 0.996 | 0.988 | |
| Pseudo-2nd-order | qe (mg g−1) | 45.25 | 30.03 | 2.14 | 1.90 |
| k2 (g mg−1 h−1) | 0.084 | 0.113 | 1.442 | 1.169 | |
| R2 | 0.995 | 0.988 | 0.950 | 0.844 | |
| Effect | kapp, ANI (h−1) | t1/2, ANI (h) | R2 | kapp, BTH (h−1) | t1/2, BTH (h) | R2 | Irradiation Dose (W m−2) |
|---|---|---|---|---|---|---|---|
| pH1 | |||||||
| 3.0 | 0.87 | 0.79 | 0.98 | 0.52 | 1.33 | 0.99 | 155.8 |
| 7.0 | 1.31 | 0.53 | 0.99 | 0.77 | 0.90 | 0.99 | 155.8 |
| 9.0 | 1.75 | 0.39 | 0.99 | 1.04 | 0.66 | 0.99 | 155.8 |
| MnO2/GAC-3 dosage2(g L−1) | |||||||
| 0.5 | 1.05 | 0.66 | 0.99 | 0.61 | 1.12 | 0.98 | 199.6 |
| 0.9 | 1.75 | 0.39 | 0.99 | 1.04 | 0.66 | 0.99 | 155.8 |
| 3.0 | 1.50 | 0.46 | 0.99 | 0.87 | 0.79 | 0.99 | 72.4 |
| Process | Pollutant | k | Treated Volume (L) | Pele (kW) | t90 (h) | ε (kW L−1) | V (L) | V × ε (kW) | Cost (€ m−3) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| FBR-Fenton | ANI | 1.739 h−1 | 0.859 | 0.012 | 1.32 | 0.014 | 238.26 | 3.33 | 0.29 | [5] |
| Ozone | ANI | 2.003 h−1 | 1.0 | 0.200 | 1.14 | 235.43 | 1.0 | 235.43 | 0.81 | [19] |
| Ozone | BTH | 1.296 h−1 | 5.0 | 0.200 | 1.77 | 1.84 | 5.0 | 9.20 | 2.76 | [9] |
| Photocatalysis | ANI and BTH mixture | 0.341 h−1 (ANI) 0.091 h−1 (BTH) | 2.15 | 0.026 | 6.75 (ANI) 25.30 (BTH) | 4.97 | 16.0 | 79.59 | 2.19 | [21] |
| FBR-Photocatalytic | ANI and BTH mixture | 1.75 h−1 (ANI) 1.04 h−1 (BTH) | 1.63 | 0.025 | 1.31 (ANI) 2.21 (BTH) | 0.057 | 10.0 | 0.57 | 0.17 1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J.; Zúñiga, V.; Rituerto, J.M. Removal of Aniline and Benzothiazole Wastewaters Using an Efficient MnO2/GAC Catalyst in a Photocatalytic Fluidised Bed Reactor. Materials 2021, 14, 5207. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14185207
Ferreiro C, Villota N, Lombraña JI, Rivero MJ, Zúñiga V, Rituerto JM. Removal of Aniline and Benzothiazole Wastewaters Using an Efficient MnO2/GAC Catalyst in a Photocatalytic Fluidised Bed Reactor. Materials. 2021; 14(18):5207. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14185207
Chicago/Turabian StyleFerreiro, Cristian, Natalia Villota, José Ignacio Lombraña, María J. Rivero, Verónica Zúñiga, and José Miguel Rituerto. 2021. "Removal of Aniline and Benzothiazole Wastewaters Using an Efficient MnO2/GAC Catalyst in a Photocatalytic Fluidised Bed Reactor" Materials 14, no. 18: 5207. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14185207