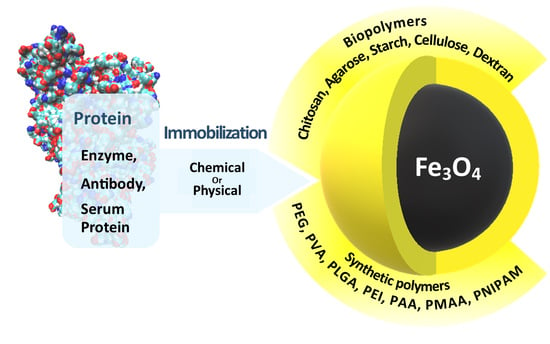
Polymer-Coated Magnetite Nanoparticles for Protein Immobilization
Abstract
:1. Introduction
2. Synthesis of Magnetite Nanoparticles—Chemical Methods
2.1. Co-Precipitation Reaction
2.2. Thermal Decomposition
2.3. Microemulsion Method
2.4. Hydrothermal Method
2.5. Sonochemical Processing
2.6. Electrochemical Methods
3. Modification of Bare Magnetite Nanoparticles
3.1. Organic Surfactants
3.2. Inorganic Compounds
3.3. Polymers and Bioactive Molecules
4. Protein Immobilization Methods
4.1. Adsorption of Protein on MNPs Surface
4.2. Covalent Binding of Protein on MNPs Surface
5. Immobilization of Proteins on Polymer-Coated Nanoparticles
5.1. Immobilization of Proteins on Nanoparticles Coated with Synthetic Polymers
5.1.1. Immobilization of Proteins on Nanoparticles Coated with Polyethylene Glycol (PEG)
5.1.2. Immobilization of Proteins on Nanoparticles Coated with Polyvinyl Alcohol (PVA)
5.1.3. Immobilization of Proteins on Nanoparticles Coated with poly(D,L-lactide-co-glycolide) (PLGA)
5.1.4. Immobilization of Proteins on Nanoparticles Coated with Polyethyleneimine (PEI)
5.1.5. Immobilization of Proteins on Nanoparticles Coated with Polyacrylic acid (PAA)
5.1.6. Immobilization of Proteins on Nanoparticles Coated with Poly(methacrylic acid) (PMAA)
5.1.7. Immobilization of Proteins on Nanoparticles Coated with poly(N-isopropylacrylamide) (PNIPAM)
5.2. Immobilization of Proteins on Nanoparticles Coated with Natural Polymers
5.2.1. Immobilization of Proteins on Nanoparticles Coated with Chitosan (CS)
5.2.2. Immobilization of Proteins on Nanoparticles Coated with Agarose
5.2.3. Immobilization of Proteins on Nanoparticles Coated with Starch
5.2.4. Immobilization of Proteins on Nanoparticles Coated with Cellulose
5.2.5. Immobilization of Proteins on Nanoparticles Coated with Dextran
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aseri, A.; Garg, S.K.; Nayak, A.; Trivedi, S.K.; Ahsan, J. Magnetic nanoparticles: Magnetic nano-technology using biomedical applications and future prospects. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 119–131. [Google Scholar]
- Dzhardimalieva, G.I.; Pomogailo, A.D.; Rozenberg, A.S.; Leonowicz, M. Magnetic Metallopolymer Nanocomposites: Preparation and Properties. Magn. Nanoparticles 2009, 59–85. [Google Scholar] [CrossRef]
- Kolesnichenko, V.L. Synthesis of Nanoparticulate Magnetic Materials; Wiley-VCH: Weinheim, Germany, 2009; ISBN 9783527407903. [Google Scholar]
- Vedmedenko, E. Competing Interactions and Patterns in Nanoworld. The Chemistry of Nanomaterials Nanoparticles Introduction to Nanotechnology; Wiley-VCH: Weinheim, Germany, 2007; ISBN 9783527404841. [Google Scholar]
- Can, M.M.; Coşkun, M.; Firat, T. A comparative study of nanosized iron oxide particles: Magnetite (Fe3O4), maghemite (γ-Fe2O3) and hematite (α-Fe2O3), using ferromagnetic resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Ab Rahman, M.Z.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Kong, J. Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J. Hazard. Mater. 2011, 193, 325–329. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zeng, J.; Gao, Y.; Tang, Z. Superparamagnetic nano-immunobeads toward food safety insurance. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Rahim, S.; Iftikhar, F.J.; Malik, M.I. Biomedical Applications of Magnetic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–328. ISBN 9780128169605. [Google Scholar]
- Magro, M.; Venerando, A.; Macone, A.; Canettieri, G.; Agostinelli, E.; Vianello, F. Nanotechnology-based strategies to develop new anticancer therapies. Biomolecules 2020, 10, 735. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Biomedical applications of advanced multifunctional magnetic nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Banerjee, R.; Katsenovich, Y.; Lagos, L.; McIintosh, M.; Zhang, X.; Li, C.-Z. Nanomedicine: Magnetic Nanoparticles and their Biomedical Applications. Curr. Med. Chem. 2010, 17, 3120–3141. [Google Scholar] [CrossRef] [PubMed]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Rehse, K. Kurzmi tteilung Vergleichende Untersuchungen zur Bindung nichtsteroidaler Antirheumatika an Humanserumalbumin und deren Interaktion rnit Phenprocoumon. Archiv der Pharmazie 1989, 7, 241–243. [Google Scholar]
- Holm, J.; Babol, L.N.; Markova, N.; Lawaetz, A.J.; Hansen, S.I. The interrelationship between ligand binding and thermal unfolding of the folate binding protein. the role of self-association and pH. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 512–519. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Rehm, F.B.H.; Chen, S.; Rehm, B.H.A. Enzyme engineering for in situ immobilization. Molecules 2016, 21, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoarau, M.; Badieyan, S.; Marsh, E.N.G. Immobilized enzymes: Understanding enzyme-surface interactions at the molecular level. Org. Biomol. Chem. 2017, 15, 9539–9551. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Knez, Ž.; Vasić, K. Micro-and Nanocarriers for Immobilization of Enzymes. In Micro and Nanotechnologies for Biotechnology; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Philippova, O.; Barabanova, A.; Molchanov, V.; Khokhlov, A. Magnetic polymer beads: Recent trends and developments in synthetic design and applications. Eur. Polym. J. 2011, 47, 542–559. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yurkov, G.Y.; Gubin, S.P.; Ovchenkov, E.A. Magnetic Nanocomposites Based on the Metal-Containing (Fe, Co, Ni) Nanoparticles inside the Polyethylene Matrix. Magn. Nanoparticles 2009, 87–115. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Dos Santos, L.A.; de Almeida, J.M.; Krieger, N.; Mateo, C. Recent trends in biomaterials for immobilization of lipases for application in non-conventional media. Catalysts 2020, 10, 697. [Google Scholar] [CrossRef]
- Bezerra, C.S.; De Farias Lemos, C.M.G.; De Sousa, M.; Gonçalves, L.R.B. Enzyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J. Appl. Polym. Sci. 2015, 132, 1–15. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Khan, U.S.; Khattak, N.S.; Rahman, A.; Khan, F. Historical development of magnetite nanoparticles synthesis. J. Chem. Soc. Pakistan 2011, 33, 793–804. [Google Scholar]
- Benelmekki, M.; Benelmekki, M. An introduction to nanoparticles and nanotechnology. Des. Hybrid Nanoparticles 2014, 2017. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T.; Matijević, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic Review of the Preparation Techniques of Iron Oxide Magnetic Nanoparticles. Nanosci. Nanotechnol. 2013, 2, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Lai, K.L.; Hu, H.; Zeng, X.B.; Lan, F.; Liu, K.X.; Wu, Y.; Gu, Z.W. The effect of [Fe3+]/[Fe2+] molar ratio and iron salts concentration on the properties of superparamagnetic iron oxide nanoparticles in the water/ethanol/toluene system. J. Nanoparticle Res. 2011, 13, 5135–5145. [Google Scholar] [CrossRef]
- Khurshid, H.; Li, W.; Chandra, S.; Phan, M.H.; Hadjipanayis, G.C.; Mukherjee, P.; Srikanth, H. Mechanism and controlled growth of shape and size variant core/shell FeO/Fe3O4 nanoparticles. Nanoscale 2013, 5, 7942–7952. [Google Scholar] [CrossRef]
- Fried, T.; Shemer, G.; Markovich, G. Ordered two-dimensional arrays of ferrite nanoparticles. Adv. Mater. 2001, 13, 1158–1161. [Google Scholar] [CrossRef]
- Kassabova-Zhetcheva, V.D.; Pavlova, L.P.; Samuneva, B.I.; Cherkezova-Zheleva, Z.P.; Mitov, I.G.; Mikhov, M.T. Characterization of superparamagnetic MgxZn1-x Fe2O4 powders. Cent. Eur. J. Chem. 2007, 5, 107–117. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Alonso, J.; Barandiarán, J.M.; Fernández Barquín, L.; García-Arribas, A. Magnetic Nanoparticles, Synthesis, Properties, and Applications; El-Gendy, J.M., Barandiarán, J.M., Hadimani, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. ISBN 9780128139059. [Google Scholar]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles as microreactors. J. Phys. Chem. 1993, 97, 6961–6973. [Google Scholar] [CrossRef]
- Chin, A.B.; Yaacob, I.I. Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart’s procedure. J. Mater. Process. Technol. 2007, 191, 235–237. [Google Scholar] [CrossRef]
- Biehl, P.; von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Baker, I. Magnetic Nanoparticle Synthesis; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081007167. [Google Scholar]
- Tang, B.; Yuan, L.; Shi, T.; Yu, L.; Zhu, Y. Preparation of nano-sized magnetic particles from spent pickling liquors by ultrasonic-assisted chemical co-precipitation. J. Hazard. Mater. 2009, 163, 1173–1178. [Google Scholar] [CrossRef]
- Wang, Y.; Nkurikiyimfura, I.; Pan, Z. Sonochemical Synthesis of Magnetic Nanoparticles. Chem. Eng. Commun. 2015, 202, 616–621. [Google Scholar] [CrossRef]
- Mathew, D.S.; Juang, R.S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Sonochemical Synthesis of Nanosized Hollow Hematite. J. Am. Chem. Soc. 2007, 129, 2242–2243. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, R.; Koltypin, Y.; Felner, I.; Gedanken, A. Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater. Sci. Eng. A 2000, 286, 101–105. [Google Scholar] [CrossRef]
- Weng, Y.C.; Rusakova, I.A.; Baikalov, A.; Chen, J.W.; Wu, N.L. Microstructural evolution of nanocrystalline magnetite synthesized by electrocoagulation. J. Mater. Res. 2005, 20, 75–80. [Google Scholar] [CrossRef]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar] [CrossRef]
- Ibrahim, M.; Serrano, K.G.; Noe, L.; Garcia, C.; Verelst, M. Electro-precipitation of magnetite nanoparticles: An electrochemical study. Electrochim. Acta 2009, 55, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Franger, S.; Berthet, P.; Berthon, J. Electrochemical synthesis of Fe3O4 nanoparticles in alkaline aqueous solutions containing complexing agents. J. Solid State Electrochem. 2004, 8, 218–223. [Google Scholar] [CrossRef]
- Mosivand, S.; Monzon, L.M.A.; Ackland, K.; Kazeminezhad, I.; Coey, J.M.D. The effect of organics on the structure and magnetization of electro-synthesised magnetite nanoparticles. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Fajaroh, F.; Setyawan, H.; Sutrisno; Nazriati; Wonorahardjo, S. To enhance the purity and crystallinity of magnetite nanoparticles prepared by surfactant-free electrochemical method by imposing higher voltage. AIP Conf. Proc. 2014, 1586, 179–182. [Google Scholar] [CrossRef]
- Marques, R.F.C.; Garcia, C.; Lecante, P.; Ribeiro, S.J.L.; Noé, L.; Silva, N.J.O.; Amaral, V.S.; Millán, A.; Verelst, M. Electro-precipitation of Fe3O4 nanoparticles in ethanol. J. Magn. Magn. Mater. 2008, 320, 2311–2315. [Google Scholar] [CrossRef]
- Marín, T.; Ortega, D.; Montoya, P.; Arnache, O.; Calderón, J. A new contribution to the study of the electrosynthesis of magnetic nanoparticles: The influence of the supporting electrolyte. J. Appl. Electrochem. 2014, 44, 1401–1410. [Google Scholar] [CrossRef]
- Xu, J.K.; Zhang, F.F.; Sun, J.J.; Sheng, J.; Wang, F.; Sun, M. Bio and nanomaterials based on Fe3O4. Molecules 2014, 19, 21506–21528. [Google Scholar] [CrossRef]
- Xia, T.; Wang, J.; Wu, C.; Meng, F.; Shi, Z.; Lian, J.; Feng, J.; Meng, J. Novel complex-coprecipitation route to form high quality triethanolamine-coated Fe3O4 nanocrystals: Their high saturation magnetizations and excellent water treatment properties. CrystEngComm 2012, 14, 5741–5744. [Google Scholar] [CrossRef]
- Muthiah, M.; Park, I.K.; Cho, C.S. Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol. Adv. 2013, 31, 1224–1236. [Google Scholar] [CrossRef]
- Gautam, A.; Van Veggel, F.C.J.M. Synthesis of nanoparticles, their biocompatibility, and toxicity behavior for biomedical applications. J. Mater. Chem. B 2013, 1, 5186–5200. [Google Scholar] [CrossRef] [PubMed]
- Kydralieva, K.A.; Dzhardimalieva, G.I.; Yurishcheva, A.A.; Jorobekova, S.J. Nanoparticles of Magnetite in Polymer Matrices: Synthesis and Properties. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1212–1230. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, L.; Sukenik, C.N.; Markovich, G.; Gan, R. Alkyl Phosphonate/Phosphate Coating on Magnetite Nanoparticles: A Comparison with Fatty Acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, J.F.; Teng, X.; Lin, X.Z.; Yang, H. “Pulling” Nanoparticles into Water: Phase Transfer of Oleic Acid Stabilized Monodisperse Nanoparticles into Aqueous Solutions of α-Cyclodextrin. Nano Lett. 2003, 3, 1555–1559. [Google Scholar] [CrossRef]
- Luchini, A.; Heenan, R.K.; Paduano, L.; Vitiello, G. Functionalized SPIONs: The surfactant nature modulates the self-assembly and cluster formation. Phys. Chem. Chem. Phys. 2016, 18, 18441–18449. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, H.; Zhuang, J.; Liu, Q. Magnetic γ-Fe2O3, Fe3O4, and Fe nanoparticles confined within ordered mesoporous carbons as efficient microwave absorbers. Phys. Chem. Chem. Phys. 2015, 17, 3802–3812. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Zhang, Q.; Zheng, Z.; Wang, L.-S.; Peng, D.L. Facile synthesis of Fe3O4/C composites for broadband microwave absorption properties. Appl. Surf. Sci. 2018, 445, 82–88. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chemie Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Autenrieth, T.; Hempelmann, R. Core shell particles consisting of cobalt ferrite and silica as model ferrofluids [CoFe2O4-SiO2 core shell particles]. J. Magn. Magn. Mater. 2002, 252, 4–6. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Carvalho, F.; Fernandes, E. Iron Oxide Nanoparticles: An Insight into their Biomedical Applications. Curr. Med. Chem. 2015, 22, 1808–1828. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E.; et al. In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells. Evaluation of nanoparticle interference with viability tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Tsourkas, A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials 2008, 29, 3583–3590. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.M.; Wang, Y.X.J.; Leung, K.C.F.; Lee, S.F.; Zhao, F.; Wang, D.W.; Lai, J.M.Y.; Wan, C.; Cheng, C.H.K.; Ahuja, A.T. Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Ling, W.; Wang, M.; Xiong, C.; Xie, D.; Chen, Q.; Chu, X.; Qiu, X.; Li, Y.; Xiao, X. Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J. Mater. Res. 2019, 34, 1828–1844. [Google Scholar] [CrossRef]
- Lee, M.H.; Leu, C.C.; Lin, C.C.; Tseng, Y.F.; Lin, H.Y.; Yang, C.N. Gold-decorated magnetic nanoparticles modified with hairpin-shaped DNA for fluorometric discrimination of single-base mismatch DNA. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A.L. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, S.; Ren, F.; Xiao, X.; Zhou, J.; Jiang, C. Controlled synthesis of magnetic iron oxides@SnO 2 quasi-hollow core-shell heterostructures: Formation mechanism, and enhanced photocatalytic activity. Nanoscale 2011, 3, 4676–4684. [Google Scholar] [CrossRef]
- Li, S.K.; Huang, F.Z.; Wang, Y.; Shen, Y.H.; Qiu, L.G.; Xie, A.J.; Xu, S.J. Magnetic Fe3O4@C@Cu2O composites with bean-like core/shell nanostructures: Synthesis, properties and application in recyclable photocatalytic degradation of dye pollutants. J. Mater. Chem. 2011, 21, 7459–7466. [Google Scholar] [CrossRef]
- Saffari, J.; Mir, N.; Ghanbari, D.; Khandan-Barani, K.; Hassanabadi, A.; Hosseini-Tabatabaei, M.R. Sonochemical synthesis of Fe3O4/ZnO magnetic nanocomposites and their application in photo-catalytic degradation of various organic dyes. J. Mater. Sci. Mater. Electron. 2015, 26, 9591–9599. [Google Scholar] [CrossRef]
- Joseph, J.; Nishad, K.K.; Sharma, M.; Gupta, D.K.; Singh, R.R.; Pandey, R.K. Fe3O4 and CdS based bifunctional core-shell nanostructure. Mater. Res. Bull. 2012, 47, 1471–1477. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, L.; Zhu, H.Y.; Shi, X.W. Studies on interaction and illumination damage of CS-Fe3O4@ZnS:Mn to bovine serum albumin. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Wang, X.; Gu, Z.; Hu, Y. Synthesis of Fe3O4@PbS hybrid nanoparticles through the combination of surface-initiated atom transfer radical polymerization and acidolysis by H 2S. J. Nanosci. Nanotechnol. 2011, 11, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chai, F.; Zhang, L.; Wang, C.; Li, L.; Liu, X.; Su, Z. Facile and fast synthesis of urchin-shaped Fe3O4@Bi2S3 core-shell hierarchical structures and their magnetically recyclable photocatalytic activity. J. Mater. Chem. 2012, 22, 4832–4836. [Google Scholar] [CrossRef]
- Rădulescu, M.; Andronescu, E.; Holban, A.M.; Vasile, B.S.; Iordache, F.; Mogoantă, L.; Dan Mogoșanu, G.; Grumezescu, A.M.; Georgescu, M.; Chifiriuc, M.C. Antimicrobial nanostructured bioactive coating based on Fe3O4 and patchouli oil for wound dressing. Metals 2016, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Kievit, F.M.; Kant, R.J.; Lin, G.; Jeon, M.; Zhang, M. Anti-HER2/neu peptide-conjugated iron oxide nanoparticles for targeted delivery of paclitaxel to breast cancer cells. Nanoscale 2015, 7, 18010–18014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Manjili, H.K. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharisov, B.I.; Eldin, M.S.M. Enzyme Immobilization: Nanopolymers for Enzyme Immobilization Applications. CRC Concise Encycl. Nanotechnol. 2018, 220–228. [Google Scholar] [CrossRef]
- Nelson, J.M.; Griffin, E.G. Adsorption of invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Cao, L. Carrier-bound Immobilized Enzymes: Principles, Application and Design. Carrier-bound Immobil. Enzym. Princ. Appl. Des. 2006, 1–563. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Sardar, M. Enzyme Immobilization: An Overview on Nanoparticles as Immobilization Matrix. Biochem. Anal. Biochem. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.M. Magnetically recoverable nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef] [PubMed]
- Nisha, S.; Karthick, S.A.; Gobi, N. A Review on Methods, Application and Properties of Immobilized Enzyme. Chem. Sci. Rev. Lett. 2012, 1, 148–155. [Google Scholar]
- Rao, S.V.; Anderson, K.W.; Bachas, L.G. Oriented Immobilization of Proteins. Mikrochim. Acta 1998, 128, 127–143. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, T.S.; Mou, C.Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Yiitolu, M.; Temoçin, Z. Immobilization of Candida rugosa lipase on glutaraldehyde-activated polyester fiber and its application for hydrolysis of some vegetable oils. J. Mol. Catal. B Enzym. 2010, 66, 130–135. [Google Scholar] [CrossRef]
- Lai, B.H.; Yeh, C.C.; Chen, D.H. Surface modification of iron oxide nanoparticles with polyarginine as a highly positively charged magnetic nano-adsorbent for fast and effective recovery of acid proteins. Process Biochem. 2012, 47, 799–805. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases–A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Wickramathilaka, M.P.; Tao, B.Y. Characterization of covalent crosslinking strategies for synthesizing DNA-based bioconjugates. J. Biol. Eng. 2019, 13, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Vashist, S.K.; Zhang, B.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Sulfo-N-hydroxysuccinimide interferes with bicinchoninic acid protein assay. Anal. Biochem. 2011, 417, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Bart, J.; Tiggelaar, R.; Yang, M.; Schlautmann, S.; Zuilhof, H.; Gardeniers, H. Room-temperature intermediate layer bonding for microfluidic devices. Lab Chip 2009, 9, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Rusli, H.; Nath, A.; Sikder, J.; Bhattacharjee, C.; Curcio, S.; Drioli, E. Immobilized biocatalytic process development and potential application in membrane separation: A review. Crit. Rev. Biotechnol. 2016, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–225. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Loboa, E.G. Nanofibrous Smart Bandages for Wound Care; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 2, ISBN 9780081006061. [Google Scholar]
- Kim, J.E.; Shin, J.Y.; Cho, M.H. Magnetic nanoparticles: An update of application for drug delivery and possible toxic eVects. Arch. Toxicol. 2012, 86, 685–700. [Google Scholar] [CrossRef]
- Tai, M.F.; Lai, C.W.; Abdul Hamid, S.B. Facile Synthesis Polyethylene Glycol Coated Magnetite Nanoparticles for High Colloidal Stability. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, A.; Joshi, N.; Chattopadhyay, K.; De, G. A facile synthesis of PEG-coated magnetite (Fe3O4) nanoparticles and their prevention of the reduction of cytochrome C. ACS Appl. Mater. Interfaces 2012, 4, 142–149. [Google Scholar] [CrossRef]
- Chang, M.; Chang, Y.J.; Chao, P.Y.; Yu, Q. Exosome purification based on peg-coated Fe3O4 Nanoparticles. PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, L.B.H.; Wulandari, I.O.; Santjojo, D.H.; Sabarudin, A. Synthesis and Characterization of Fe3O4 Nanoparticles with Polyvinyl Alcohol (PVA) as Capping Agent and Glutaraldehyde as Crosslinkers. Nature B 2018, 4, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, M.; Akhtar, M.S.; Shaari, A.; Riaz, S.; Naseem, S.; Masood, M.; Saeed, M.A. Magnetic properties of polyvinyl alcohol and doxorubicine loaded iron oxide nanoparticles for anticancer drug delivery applications. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Shokrgozar, M.A.; Sardari, S.; Moghadam, M.K.; Vali, H.; Laurent, S.; Stroeve, P. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale 2011, 3, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Laochai, T.; Mooltongchun, M.; Teepoo, S. Design and Construction of Magnetic Nanoparticles Incorporated with a Chitosan and Poly (vinyl) Alcohol Cryogel and its Application for Immobilization of Horseradish Peroxidase. Energy Procedia 2016, 89, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Tahir, M.N.; Siwy, Z.; Neumann, R.; Tremel, W.; Ensinger, W. Hydrogen peroxide sensing with horseradish peroxidase-modified polymer single conical nanochannels. Anal. Chem. 2011, 83, 1673–1680. [Google Scholar] [CrossRef]
- Sahin, S.; Ozmen, I. Covalent immobilization of trypsin on polyvinyl alcohol-coated magnetic nanoparticles activated with glutaraldehyde. J. Pharm. Biomed. Anal. 2020, 184, 113195. [Google Scholar] [CrossRef]
- Gerlt, J.A. Stabilization of Reactive Intermediates and Transition States in Enzyme Active Sites by Hydrogen Bonding. Compr. Nat. Prod. Chem. 1999, 5–29. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2011, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hirenkumar, M.; Steven, S. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Review poly(lactic-co-glycolic acid): Applications and future prospects for periodontal tissue regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Bianco, J.; Danhier, P.; Zhao, M.; Bastiancich, C.; Gallez, B.; Danhier, F.; Préat, V. Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma. Int. J. Nanomed. 2018, 13, 4509–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spillmann, C.M.; Naciri, J.; Algar, W.R.; Medintz, I.L.; Delehanty, J.B. Multifunctional liquid crystal nanoparticles for intracellular fluorescent imaging and drug delivery. ACS Nano 2014, 8, 6986–6997. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Leary, J.F. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int. J. Nanomed. 2014, 9, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, C.H.; Park, J.; Seo, S.; Lim, E.K.; Song, Y.J.; Suh, J.S.; Yoon, H.G.; Huh, Y.M.; Haam, S. Antibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancer. J. Mater. Chem. 2007, 17, 2695–2699. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.H.; Tóth, J.; Gyenis, J.; Feczkó, T. Surface modification of HSA containing magnetic PLGA nanoparticles by poloxamer to decrease plasma protein adsorption. Colloids Surfaces B Biointerfaces 2014, 122, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Ling, M.; Jia, M.; Huang, P.; Li, C.; Yan, B. Facile synthesis and characterization of polyethylenimine-coated Fe3O4 superparamagnetic nanoparticles for cancer cell separation. Mol. Med. Rep. 2014, 9, 1080–1084. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, L.; Ma, Y.; Li, X.; Gu, H. Control of aggregate size of polyethyleneimine-coated magnetic nanoparticles for magnetofection. Nano Res. 2009, 2, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Kwolek, U.; Wójcik, K.; Janiczek, M.; Nowakowska, M.; Kepczynski, M. Synthesis and antibacterial properties of quaternary ammonium derivative of polyethylenimine. Polimery 2017, 62, 311–315. [Google Scholar] [CrossRef]
- Xia, T.T.; Liu, C.Z.; Hu, J.H.; Guo, C. Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem. Eng. J. 2016, 295, 201–206. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Perazzini, R.; Saladino, R. Oxidative functionalisation of lignin by layer-by-layer immobilised laccases and laccase microcapsules. Appl. Catal. A Gen. 2010, 372, 115–123. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.; Meister, M.; Hussy, S.; Cordes, A.; Enderle, G.; Saningong, A.; Schauer, F. Enhanced laccase-mediated transformation of diclofenac and flufenamic acid in the presence of bisphenol A and testing of an enzymatic membrane reactor. AMB Express 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Zuvin, M.; Kuruoglu, E.; Kaya, V.O.; Unal, O.; Kutlu, O.; Yagci Acar, H.; Gozuacik, D.; Kosar, A. Magnetofection of green fluorescent protein encoding DNA-bearing polyethyleneimine-coated superparamagnetic iron oxide nanoparticles to human breast cancer cells. ACS Omega 2019, 4, 12366–12374. [Google Scholar] [CrossRef] [Green Version]
- Wiogo, H.T.R.; Lim, M.; Bulmus, V.; Gutiérrez, L.; Woodward, R.C.; Amal, R. Insight into serum protein interactions with functionalized magnetic nanoparticles in biological media. Langmuir 2012, 28, 4346–4356. [Google Scholar] [CrossRef] [Green Version]
- Kannan, K.; Mukherjee, J.; Gupta, M.N. Use of polyethyleneimine coated Fe3O4 nanoparticles as an ion-exchanger for protein separation. Sci. Adv. Mater. 2013, 5, 1477–1484. [Google Scholar] [CrossRef]
- Gräfe, C.; Weidner, A.; Lühe, M.V.D.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle-cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The effect of surface charge of functionalized Fe3O4 nanoparticles on protein adsorption and cell uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef] [Green Version]
- Roohi, F.; Lohrke, J.; Ide, A.; Schütz, G.; Dassler, K. Studying the effect of particle size and coating type on the blood kinetics of superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2012, 7, 4447–4458. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhuang, L.; Lin, H.; Shen, H.; Li, J.W. Preparation and characterization of polyacrylic acid coated magnetite nanoparticles functionalized with amino acids. Thin Solid Films 2013, 544, 368–373. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic acid-coated iron oxide magnetic nanoparticles: The polymer molecular weight influence. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 543, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Hamidreza, S. Synthesis of Nanocomposition of Poly Acrylic Acid/Chitosan Coated-Magnetite Nanoparticles to Investigation of Interaction with BSA and IGG Proteins. Int. J. Nanomater. Nanotechnol. Nanomed. 2017, 3, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.H.; Liao, M.H.; Chen, D.H. Fast and efficient recovery of lipase by polyacrylic acid-coated magnetic nano-adsorbent with high activity retention. Sep. Purif. Technol. 2006, 51, 113–117. [Google Scholar] [CrossRef]
- Liao, M.H.; Chen, D.H. Preparation and characterization of a novel magnetic nano-adsorbent. J. Mater. Chem. 2002, 12, 3654–3659. [Google Scholar] [CrossRef]
- Ma, Y.H.; Wu, S.Y.; Wu, T.; Chang, Y.J.; Hua, M.Y.; Chen, J.P. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials 2009, 30, 3343–3351. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, K.; Gu, H. Investigations on the interactions of proteins with polyampholyte-coated magnetite nanoparticles. J. Phys. Chem. B 2013, 117, 14129–14135. [Google Scholar] [CrossRef]
- Xiao, W.; Gu, H.; Li, D.; Chen, D.; Deng, X.; Jiao, Z.; Lin, J. Microwave-assisted synthesis of magnetite nanoparticles for MR blood pool contrast agents. J. Magn. Magn. Mater. 2012, 324, 488–494. [Google Scholar] [CrossRef]
- Woźniak, E.; Špírková, M.; Šlouf, M.; Garamus, V.M.; Šafaříková, M.; Šafařík, I.; Štěpánek, M. Stabilization of aqueous dispersions of poly(methacrylic acid)-coated iron oxide nanoparticles by double hydrophilic block polyelectrolyte poly(ethylene oxide)-block-poly(N-methyl-2-vinylpyridinium iodide). Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.; Ahmad, A.; Islam, S.S. Magnetic Fe3O4@poly(methacrylic acid) particles for selective preconcentration of trace arsenic species. Microchim. Acta 2017, 184, 2007–2014. [Google Scholar] [CrossRef]
- Li, K.; Chen, K.; Wang, Q.; Zhang, Y.; Gan, W. Synthesis of poly(acrylic acid) coated magnetic nanospheres via a multiple polymerization route. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Mekseriwattana, W.; Srisuk, S.; Kriangsaksri, R.; Niamsiri, N.; Prapainop, K. The Impact of Serum Proteins and Surface Chemistry on Magnetic Nanoparticle Colloidal Stability and Cellular Uptake in Breast Cancer Cells. AAPS PharmSciTech 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chow, C.M. Carboxyl group (-CO2H) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applications. J. Mater. Chem. 2004, 14, 2781–2786. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Alipour, A.; Shekardasht, M.B.; Gharbani, P. The synthesis, characterization and applications of poly[:N-isopropylacrylamide-co-3-allyloxy-1,2-propanediol] grafted onto modified magnetic nanoparticles. RSC Adv. 2020, 10, 3511–3519. [Google Scholar] [CrossRef]
- Nguyen, N.H.A.; Darwish, M.S.A.; Stibor, I.; Kejzlar, P.; Ševců, A. Magnetic Poly(N-isopropylacrylamide) Nanocomposites: Effect of Preparation Method on Antibacterial Properties. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Shamim, N.; Hong, L.; Hidajat, K.; Uddin, M.S. Thermosensitive-polymer-coated magnetic nanoparticles: Adsorption and desorption of Bovine Serum Albumin. J. Colloid Interface Sci. 2006, 304, 1–8. [Google Scholar] [CrossRef]
- Dionigi, C.; Lungaro, L.; Goranov, V.; Riminucci, A.; Piñeiro-Redondo, Y.; Bañobre-López, M.; Rivas, J.; Dediu, V. Smart magnetic poly(N-isopropylacrylamide) to control the release of bio-active molecules. J. Mater. Sci. Mater. Med. 2014, 25, 2365–2371. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs. 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, S.N. The antiviral activity of chitosan (review). Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Siódmiak, T.; Chełminiak, D.; Cyganiuk, A.; Marszałł, M.P. Magnetic nanoparticles with surfaces modified with chitosan-poly[N-benzyl-2-(methacryloxy)-N,N-dimethylethanaminium bromide] for lipase immobilization. Appl. Surf. Sci. 2014, 288, 641–648. [Google Scholar] [CrossRef]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R,S)-ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Mylkie, K.; Kozlowska, M.; Nowak, P.; Chelminiak-Dudkiewicz, D.; Kozakiewicz, A.; Ilnicka, A.; Kaczmarek-Kedziera, A. Effect of geometrical structure, drying, and synthetic method on aminated chitosan-coated magnetic nanoparticles utility for HSA effective immobilization. Molecules 2019, 24, 1925. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Mylkie, K.; Nowak, P.; Rybczynski, P.; Sikora, A.; Chelminiak-Dudkiewicz, D.; Kaczmarek-Kedziera, A. Testing for ketoprofen binding to HSA coated magnetic nanoparticles under normal conditions and after oxidative stress. Molecules 2020, 25, 1945. [Google Scholar] [CrossRef]
- Qiao, T.; Wu, Y.; Jin, J.; Gao, W.; Xie, Q.; Wang, S.; Zhang, Y.; Deng, H. Conjugation of catecholamines on magnetic nanoparticles coated with sulfonated chitosan. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 380, 169–174. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Zhang, L.M. Bioconjugation of papain on superparamagnetic nanoparticles decorated with carboxymethylated chitosan. Biomacromolecules 2007, 8, 1480–1486. [Google Scholar] [CrossRef]
- Li, G.Y.; Jiang, Y.R.; Huang, K.L.; Ding, P.; Yao, L.L. Kinetics of adsorption of Saccharomyces cerevisiae mandelated dehydrogenase on magnetic Fe3O4-chitosan nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 320, 11–18. [Google Scholar] [CrossRef]
- Wang, S.N.; Zhang, C.R.; Qi, B.K.; Sui, X.N.; Jiang, L.Z.; Li, Y.; Wang, Z.J.; Feng, H.X.; Wang, R.; Zhang, Q.Z. Immobilized alcalase alkaline protease on the magnetic chitosan nanoparticles used for soy protein isolate hydrolysis. Eur. Food Res. Technol. 2014, 239, 1051–1059. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr. Polym. 2017, 157, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M.P. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization—new type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Siódmiak, T.; Tarczykowska, A.; Sroka, W.D.; Ziegler-Borowska, M.; Marszałł, M.P. Enantioselective acetylation of (R,S)-atenolol: The use of Candida rugosa lipases immobilized onto magnetic chitosan nanoparticles in enzyme-catalyzed biotransformation. J. Mol. Catal. B Enzym. 2016, 134, 43–50. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Kim, S.M.; Sayed, M.; Younesi, H.; Bahramifar, N.; Park, J.H.; Pyo, S.H. Lipase-immobilized chitosan-crosslinked magnetic nanoparticle as a biocatalyst for ring opening esterification of itaconic anhydride. Biochem. Eng. J. 2019, 143, 141–150. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liu, Y.C.; Chang, C.M.J.; Chen, J.H.; Chang, C.; Shieh, C.J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym. 2012, 87, 2538–2545. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jiang, X.P.; Li, Y.; Zeng, S.; Zhang, Y.W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; Dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of lipase a from Candida antarctica onto Chitosan-coated magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Chełminiak, D.; Siódmiak, T.; Sikora, A.; Marszałł, M.P.; Kaczmarek, H. Synthesis of new chitosan coated magnetic nanoparticles with surface modified with long-distanced amino groups as a support for bioligands binding. Mater. Lett. 2014, 132, 63–65. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.S.; Noh, J.M.; Lee, Y.S. Amino acid modified chitosan beads: Improved polymer supports for immobilization of lipase from Candida rugosa. J. Mol. Catal. B Enzym. 2009, 57, 123–129. [Google Scholar] [CrossRef]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Ziegler-Borowska, M.; Marszałł, M.P. Enantioseparation of (RS)-atenolol with the use of lipases immobilized onto new-synthesized magnetic nanoparticles. Tetrahedron Asymmetry 2017, 28, 374–380. [Google Scholar] [CrossRef]
- Marszałł, M.P.; Sroka, W.D.; Sikora, A.; Chełminiak, D.; Ziegler-Borowska, M.; Siódmiak, T.; Moaddel, R. Ligand fishing using new chitosan based functionalized Androgen Receptor magnetic particles. J. Pharm. Biomed. Anal. 2016, 127, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Mora, V.; Soares, P.; Echeverria, C.; Hernández, R.; Mijangos, C. Composite Chitosan/Agarose Ferrogels for Potential Applications in Magnetic Hyperthermia. Gels 2015, 1, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Cheong, K.L.; Du, H. Modification and comparison of three Gracilaria spp. agarose with methylation for promotion of its gelling properties. Chem. Cent. J. 2017, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Adivi, F.G.; Hashemi, P. Ultrafine agarose-coated superparamagnetic iron oxide nanoparticles (AC-SPIONs): A promising sorbent for drug delivery applications. J. Iran. Chem. Soc. 2018, 15, 1145–1152. [Google Scholar] [CrossRef]
- Adivi, F.G.; Hashemi, P.; Tehrani, A.D. Agarose-coated Fe3O4 @SiO2 magnetic nanoparticles modified with sodium dodecyl sulfate, a new promising sorbent for fast adsorption/desorption of cationic drugs. Polym. Bull. 2019, 76, 1239–1256. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Review on Magnetic Natural Polymer Constructed Hydrogels as Vehicles for Drug Delivery. Biomacromolecules 2020. [Google Scholar] [CrossRef]
- Chen, T.; Yang, W.; Guo, Y.; Yuan, R.; Xu, L.; Yan, Y. Enhancing catalytic performance of β-glucosidase via immobilization on metal ions chelated magnetic nanoparticles. Enzyme Microb. Technol. 2014, 63, 50–57. [Google Scholar] [CrossRef]
- Klimaviciute, R.; Bendoraitiene, J.; Lekniute, E.; Zemaitaitis, A. Non-stoichiometric complexes of cationic starch and 4-sulfophthalic acid and their flocculation efficiency. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 457, 180–188. [Google Scholar] [CrossRef]
- Uthaman, S.; Lee, S.J.; Cherukula, K.; Cho, C.S.; Park, I.K. Polysaccharide-coated magnetic nanoparticles for imaging and gene therapy. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Adak, S.; Banerjee, R. A green approach for starch modification: Esterification by lipase and novel imidazolium surfactant. Carbohydr. Polym. 2016, 150, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Borowska, M. Magnetic nanoparticles coated with aminated starch for HSA immobilization- simple and fast polymer surface functionalization. Int. J. Biol. Macromol. 2019, 136, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Hussain, A.; Ramteke, A.; Sharma, H.K.; Deb, P.; Maji, T.K. Carboxymethyl starch-coated iron oxide magnetic nanoparticles: A potential drug delivery system for isoniazid. Iran. Polym. J. 2015, 24, 815–828. [Google Scholar] [CrossRef]
- Dung, T.T.; Danh, T.M.; Hoa, L.T.M.; Chien, D.M.; Duc, N.H. Structural and magnetic properties of starch-coated magnetite nanoparticles. J. Exp. Nanosci. 2009, 4, 259–267. [Google Scholar] [CrossRef]
- Zheng, M.; Lu, J.; Zhao, D. Effects of starch-coating of magnetite nanoparticles on cellular uptake, toxicity and gene expression profiles in adult zebrafish. Sci. Total Environ. 2018, 622–623, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cheng, F.; Lu, Y.; Ge, W.; Zhang, M.; Yue, B. Immobilization of pectinase from Penicillium oxalicum F67 onto magnetic cornstarch microspheres: Characterization and application in juice production. J. Mol. Catal. B Enzym. 2013, 97, 137–143. [Google Scholar] [CrossRef]
- Gagnon, P.; Toh, P.; Lee, J. High productivity purification of immunoglobulin G monoclonal antibodies on starch-coated magnetic nanoparticles by steric exclusion of polyethylene glycol. J. Chromatogr. A 2014, 1324, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shen, Y.; Xie, A.; Zhang, W. Preparation and protein immobilization of magnetic dialdehyde starch nanoparticles. J. Phys. Chem. B 2013, 117, 3720–3725. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Noguchi, Y.; Nakajima, M.; Sato, S.; Mukataka, S.; Ichikawa, S. Production of chitosan oligosaccharides using chitosanase immobilized on amylose-coated magnetic nanoparticles. Process Biochem. 2008, 43, 62–69. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Van Rie, J.; Thielemans, W. Cellulose-gold nanoparticle hybrid materials. Nanoscale 2017, 9, 8525–8554. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Berry, C.C.; West, R.M.; Gonzalez-Monterrubio, E.; Angulo-Molina, A.; Arias-Carrión, Ó.; Méndez-Rojas, M.Á. Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood-brain barrier. Nanoscale Adv. 2019, 1, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Namdeo, M.; Bajpai, S.K. Immobilization of α-amylase onto cellulose-coated magnetite (CCM) nanoparticles and preliminary starch degradation study. J. Mol. Catal. B Enzym. 2009, 59, 134–139. [Google Scholar] [CrossRef]
- Ivanova, V.; Petrova, P.; Hristov, J. Application in the Ethanol Fermentation of Immobilized Yeast Cells in Matrix of Alginate/Magnetic Nanoparticles, on Chitosan-Magnetite Microparticles and Cellulose-coated Magnetic Nanoparticles. arXiv 2011, arXiv:1105.0619. [Google Scholar]
- Anirudhan, T.S.; Rejeena, S.R.; Binusree, J. Adsorptive separation of myoglobin from aqueous solutions using iron oxide magnetic nanoparticles modified with functionalized nanocrystalline cellulose. J. Chem. Eng. Data 2013, 58, 1329–1339. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Wang, J.; Fang, G.; Liu, J.; Wang, S. Nano-crystalline cellulose-coated magnetic nanoparticles for affinity adsorption of glycoproteins. Analyst 2020, 145, 3407–3413. [Google Scholar] [CrossRef]
- Mohammadi, F.; Moeeni, M.; Li, C.; Boukherroub, R.; Szunerits, S. Interaction of cellulose and nitrodopamine coated superparamagnetic iron oxide nanoparticles with alpha-lactalbumin. RSC Adv. 2020, 10, 9704–9716. [Google Scholar] [CrossRef]
- Ohannesian, N.; De Leo, C.T.; Martirosyan, K.S. Dextran coated superparamagnetic iron oxide nanoparticles produced by microfluidic process. Mater. Today Proc. 2019, 13, 397–403. [Google Scholar] [CrossRef]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef] [PubMed]
- Hradil, J.; Pisarev, A.; Babič, M.; Horák, D. Dextran-modified iron oxide nanoparticles. China Particuology 2007, 5, 162–168. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D.D. Highly water soluble and recovered dextran coated fe3o 4 magnetic nanoparticles for brackish water desalination. Sep. Purif. Technol. 2011, 81, 392–399. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Konstantinova, E.A.; Kokorin, A.I.; Gyergyek, S.; Leitgeb, M. Structural and magnetic characteristics of carboxymethyl dextran coated magnetic nanoparticles: From characterization to immobilization application. React. Funct. Polym. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R.; Moore, A.; Mahmood, U.; Bhorade, R.; Benveniste, H.; Chiocca, E.A.; Basilion, J.P. In vivo magnetic resonance imaging of transgene expression. Nat. Med. 2000, 6, 351–354. [Google Scholar] [CrossRef]
- Hogemann, D.; Josephson, L.; Weissleder, R.; Basilion, J.P. Improvement of MRI probes to allow efficient detection of gene expression. Bioconjug. Chem. 2000, 11, 941–946. [Google Scholar] [CrossRef]
- Horng, H.E.; Yang, S.Y.; Hong, C.Y.; Liu, C.M.; Tsai, P.S.; Yang, H.C.; Wu, C.C. Biofunctionalized magnetic nanoparticles for high-sensitivity immunomagnetic detection of human C-reactive protein. Appl. Phys. Lett. 2006, 88, 1–4. [Google Scholar] [CrossRef]
- Templin, M.F.; Stoll, D.; Schrenk, M.; Traub, P.C.; Vöhringer, C.F.; Joos, T.O. Protein microarray technology. Trends Biotechnol. 2002, 20, 160–166. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Li, M.; Xia, N.; Huang, Q.; Do, H.; Liu, Y.N.; Zhou, F. Carboxymethylated dextran-coated magnetic iron oxide nanoparticles for regenerable bioseparation. J. Nanosci. Nanotechnol. 2011, 11, 10187–10192. [Google Scholar] [CrossRef] [PubMed]
- Ziv, O.; Avtalion, R.R.; Margel, S. Immunogenicity of bioactive magnetic nanoparticles: Natural and acquired antibodies. J. Biomed. Mater. Res. Part A 2008, 85, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Narain, R.; Gonzales, M.; Hoffman, A.S.; Stayton, P.S.; Krishnan, K.M. Synthesis of monodisperse biotinylated p(NIPAAm)-coated iron oxide magnetic nanoparticles and their bioconjugation to streptavidin. Langmuir 2007, 23, 6299–6304. [Google Scholar] [CrossRef] [PubMed]
























| Methods | Advantages | Disadvantages |
|---|---|---|
| Chemical Co-precipitation | simple and effective | not suitable for the preparation of high-purity accurate stoichiometric phase |
| Thermal Decomposition | particle size and shapes are controllable | time-consuming synthesis at high temperatures |
| Microemulsion | uniform properties | surfactants are difficult to remove; small amount can be synthesized |
| Hydrothermal | particle size and shapes are easily controllable homogeneity | high pressure and high temperature reaction |
| Sonochemical | size distribution in narrow particle | mechanism not still understood |
| Electrochemical | easy to control particle size | reproducibility |
| Method of Immobilization | Binding Nature | Advantages | Disadvantages |
|---|---|---|---|
| Adsorption | weak interactions such as hydrogen bond, hydrophobic, and van der Waals interactions | - does not or little affects the enzyme structure - simple, cheap, and easy - no conformational change of the protein - no need to use reagents | - low stability - non-specific adsorption |
| Protein | Bare MNPs | MNPs Coated with Polymethacrylic Acid | MNPs Coated with Linear Polyethylenimine | MNPs Coated with Branched Polyethylenimine |
|---|---|---|---|---|
| Albumin | + | + | + | + |
| Antithrombin | - | - | - | + |
| α-2-HS-glycoprotein | + | - | - | - |
| Inter- α-inhibitor | - | - | + | + |
| Apolipoprotein A-1 | - | - | + | + |
| Apolipoprotein E | - | - | + | + |
| Complement Component 4A | - | - | + | + |
| Tetranectin | + | + | - | - |
| α-fetoprotein | - | - | - | + |
| α-1-antiproteinase | - | - | - | + |
| Kininogen | - | + | - | - |
| Complement factor H | + | - | - | - |
| Hemoglobin | + | + | + | + |
| Immunoglobulin | - | + | - | - |
| Complement factor I | + | - | - | - |
| Complement factor B | + | + | - | - |
| Apolipoprotein B | + | - | - | - |
| Lactoferrin | - | + | - | - |
| Protein | Polimer | References |
|---|---|---|
| Fetal bovine serum (FBS) | polyethylene glycol (PEG) | [125] |
| Cytochrome C | polyethylene glycol (PEG) | [124] |
| Transferrin | polyvinyl alcohol (PVA) | [131] |
| Horseradish peroxidase | polyvinyl alcohol/chitosan (PVA/CS) | [132] |
| Trypsin | polyvinyl alcohol (PVA) | [134] |
| Herceptin antibody (HER) | poly(D,L-lactide-co-glycolide) (PLGA)/polyvinyl alcohol (PVA) | [143] |
| Bovine serum albumin (BSA) | poly(D,L-lactide-co-glycolide)(PLGA) | [145] |
| Laccase from Trametes versicolor | polyethyleneimine (PEI) | [149] |
| Green fluorescent protein | polyethyleneimine (PEI) | [154] |
| Fetal bovine serum (FBS) | polyethyleneimine (PEI) | [155] |
| Candida rugosa lipase i Mucor miehei lipase | polyethyleneimine (PEI) | [156] |
| Fetal bovine serum (FBS) | polyethyleneimine (PEI) | [158] |
| Fetal bovine serum (FBS) | polyacrylic acid (PAA) | [158] |
| Fetal bovine serum (FBS) | polyacrylic acid (PAA) | [155] |
| Bovine serum albumin (BSA) and Immunoglobulin G | polyacrylic acid (PAA)/chitosan (CS) | [163] |
| Lipase from Candida Rugosa | polyacrylic acid (PAA) | [164] |
| Tissue plasminogen activator (rtPA) | polyacrylic acid (PAA) | [166] |
| Lysozyme (LYZ) and bovine serum albumin (BSA) | polyacrylic acid (PAA) | [167] |
| Fetal bovine serum (FBS) | polymethacrylic acid (PMAA) | [172] |
| Bovine serum albumin (BSA) | poly(N-isopropylacrylamide) (PNIPAM) | [177] |
| Vascular endothelial growth factor (VEGF) | poly(N-isopropylacrylamide) (PNIPAM) | [178] |
| Streptavidin | poly(N-isopropylacrylamide) (PNIPAM) | [242] |
| Papain from Carica papaya | Chitosan (CS) | [188] |
| Saccharomyces cerevisiae Mandelated dehydrogenase (SCMD) | Chitosan (CS) | [189] |
| Pectinase | Chitosan (CS) | [191] |
| Lipase from Candida rugosa | Chitosan (CS) | [195] |
| Lipase from Thermomyces lanuginosus | Chitosan (CS) | [196] |
| Lipase A from Candida antarctica | Chitosan (CS) | [197] |
| Lipase B from Candida antarctica | Chitosan (CS) | [194] |
| Lipase from Candida rugosa | Chitosan (CS) | [182] |
| Lipase from Candida rugosa | Chitosan (CS) | [183] |
| Lipase from Candida rugosa and Human serum albumin (HSA) | Chitosan (CS) | [198] |
| Lipase from Candida rugosa | Chitosan (CS) | [193] |
| Lipase from Candida rugosa | Chitosan (CS) | [201] |
| Lipase from Candida rugosa | Chitosan (CS) | [192] |
| Androgen receptor (AR) | Chitosan (CS) | [202] |
| Human serum albumin (HSA) | Chitosan (CS) | [184] |
| Human serum albumin (HSA) | Chitosan (CS) | [185] |
| β-Glucosidase (BGL) | Agarose | [208] |
| Human serum albumin (HSA) | Starch | [212] |
| Pectinase | Starch | [216] |
| Immunoglobulin G | Starch | [217] |
| Bovine serum albumin (BSA) | Starch | [218] |
| Chitosanase | Amylose | [219] |
| α-amylase | Cellulose | [224] |
| α-amylase | Cellulose | [225] |
| Heme | Cellulose | [226] |
| Lysozyme | Cellulose | [227] |
| Bovine α-lactalbumin (BLA) | Cellulose | [228] |
| Transferrin | Dextran | [237] |
| Anti-CRP antibodies | Dextran | [238] |
| Anti-BSA | Dextran | [240] |
| Alcohol dehydrogenase (ADH) | Dextran | [234] |
| Human serum albumin (HSA) | Dextran | [241] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14020248
Mylkie K, Nowak P, Rybczynski P, Ziegler-Borowska M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials. 2021; 14(2):248. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14020248
Chicago/Turabian StyleMylkie, Kinga, Pawel Nowak, Patryk Rybczynski, and Marta Ziegler-Borowska. 2021. "Polymer-Coated Magnetite Nanoparticles for Protein Immobilization" Materials 14, no. 2: 248. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14020248