Thermoplastic Intumescent Coatings Modified with Pentaerythritol-Occluded Carbon Nanotubes
Abstract
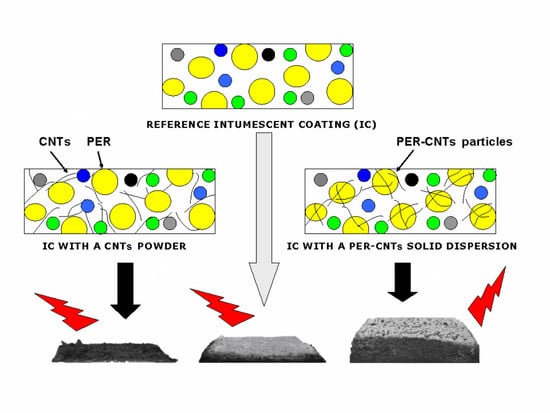
:1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- Poly(vinyl acetate) (PVAc) with the average molecular weight of 176,000 g/mol, density ca. 1.2 g/cm3, glass transition temperature ca. 42 °C, softening temperature 150 °C and solid content >99% (M50; Synthomer, Essex, UK);
- (2)
- Ammonium polyphosphate (type II) (APP), a powder with the average particles diameter of 18 µm (FR Cross 484; Budenheim, Germany);
- (3)
- Melamine (MEL), a powder with a particles size ≤40 µm (Melafine; OCI Nitrogen, Amsterdam, The Netherlands);
- (4)
- Pentaerythritol (PER), a powder with a particles size ≤40 µm, density ca. 1.4 g/cm3 (Dispersion&Resins, Włocławek, Poland);
- (5)
- Multi-walled carbon nanotubes (CNTs), a powder with a particle length ≤1.5 µm (Nanocyl NC7000; Nanocyl, Sambreville, Belgium);
- (6)
- An aqueous dispersion of Nanocyl NC7000 (3 wt.% of CNTs) (Aquacyl AQ0302; Nanocyl, Sambreville, Belgium);
- (7)
- Titanium dioxide (a rutile type) with the average particles size of 290 nm (Tytanpol R-001; GA Z.Ch. Police, Poland);
- (8)
- Zinc borate heptahydrate (ZB) with the particles size ca. 40 µm (POCh, Gliwice, Poland);
- (9)
- A wetting/dispersing additive based on hydroxyl-functional carboxylic acid ester (Disperbyk 108; BYK-Chemie, Wesel, Germany);
- (10)
- A silicone defoamer (Byk-066N; BYK-Chemie);
- (11)
- n-Butyl acetate as a solvent (Chempur, Piekary Śląskie, Poland).
2.2. Sample Preparation
2.2.1. Preparation of the Solid CNTs Dispersions in PER
2.2.2. Preparation of the Intumescent Paints and Coatings
2.3. Methods
3. Results
3.1. Furnace Test Results and Intumescent Coatings Features
3.2. Features of Charred Intumescent Coatings
4. Conclusions
- (1)
- Utilization of CNTs in the form of their solid dispersion in PER (PER-CNTs) allows to obtain intumescent solvent-based paints with an acceptable application viscosity while the CNT powder incorporation causes a significant increment of this feature as well as negatively influences the main properties of the dry coatings (i.e., thermal insulation time (TIT) and intumescent factor (IF)).
- (2)
- The coatings filled with PER-CNTs reached markedly larger TIT and IF values than a reference sample (R-0); the best thermal insulation features (+4.6 min vs. R-0) were observed for the system containing 2 wt. parts of CNTs occluded by PER (per 100 wt. parts of coating components). Slightly lower TIT values were recorded for the PER-CNTs-based samples containing 1 or 3 wt. parts of CNTs (ca. +3.3 min vs. R-0). The IF parameter increased with the increasing CNT content (+10.1 a.u. at 3 wt. parts of CNTs).
- (3)
- The PER-CNTs-based coatings created chars (during a fire test) with markedly better mechanical properties in relation to the unmodified sample; however, the highest compression strength was recorded for the system with the lowest CNT concentration.
- (4)
- CNTs in the form of the PER-CNTs solid dispersions do not affect chemical composition of the charred coatings (no differences for FTIR spectra of the reference and modified samples were observed).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vandersall, H. Intumescent coating systems, their development and chemistry. J. Fire Flamm. 1971, 2, 97–140. [Google Scholar]
- Gu, J.; Zhang, G.; Dong, S.; Zhang, Q.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers: Part I. thermal degradation of ammonium polyphosphate–pentaerythritol mixtures. Polym. Degrad. Stab. 1984, 6, 243–252. [Google Scholar] [CrossRef]
- Łopiński, J.; Schmidt, B.; Bai, Y.; Kowalczyk, K. Effect of the B:Zn:H2O molar ratio on the properties of poly(Vinyl Acetate) and zinc borate-based intumescent coating materials exposed to a quasi-real cellulosic fire. Polymers 2020, 12, 2542. [Google Scholar] [CrossRef] [PubMed]
- Staggs, J. Important parameter groups in thermal protection of polymers. Materials 2015, 18, 4679–4698. [Google Scholar] [CrossRef] [Green Version]
- Zielecka, M.; Rabajczyk, A.; Cyganczuk, K.; Pastuszka, Ł.; Jurecki, L. Silicone resin-based intumescent paints. Materials 2020, 13, 4785. [Google Scholar] [CrossRef]
- Anna, P.; Marosi, G.; Csontos, I.; Bourbigot, S.; Le Bras, S.; Delobel, R. Influence of modified rheology on the efficiency of intumescent flame retardant systems. Polym. Degrad. Stab. 2001, 74, 423–426. [Google Scholar] [CrossRef]
- Fontaine, G.; Bourbigot, S.; Duquesne, S. Neutralized flame retardant phosphorus agent: Facile synthesis, reaction to fire in pp and synergy with zinc borate. Polym. Degrad. Stab. 2008, 93, 68–76. [Google Scholar] [CrossRef]
- Tomczak, M.; Łopiński, J.; Kowalczyk, K.; Schmidt, B.; Rokicka, J. Vinyl intumescent coatings modified with platelet-type nanofillers. Prog. Org. Coat. 2019, 126, 97–105. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Zhu, X.; Sun, Z. Montmorillonite-synergized water-based intumescent flame retardant coating for plywood. Coatings 2020, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.C.; Rezende, M.J.; Nascimento, M.A.; Nascimento, R.S.; Ribeiro, S. Synergistic action of montmorillonite with an intumescent formulation: The impact of the nature and the strength of acidic sites on the flame-retardant properties of polypropylene composites. Polymers 2020, 12, 2781. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Bréant, P.; Trémillon, J.; Delobel, R. Zeolites: New synergistic agents for intumescent fire retardant thermoplastic formulations—Criteria for the choice of the zeolite. Fire Mater. 1996, 20, 145–154. [Google Scholar] [CrossRef]
- Rao, T.; Naidu, T.; Kim, M.; Parvatamma, B.; Prashanthi, Y.; Koo, B. Influence of zinc oxide nanoparticles and char forming agent polymer on flame retardancy of intumescent flame retardant coatings. Nanomaterials 2020, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Hu, Z.; Zhang, S.; Gu, X.; Wang, H.; Jiang, P.; Zhao, Q. Effects of titanium dioxide on the flammability and char formation of water-based coatings containing intumescent flame retardants. Prog. Org. Coat. 2015, 78, 318–324. [Google Scholar] [CrossRef]
- Lin, M.; Li, B.; Li, Q.; Li, S.; Zhang, S. Synergistic effect of metal oxides on the flame retardancy and thermal degradation of novel intumescent flame-retardant thermoplastic polyurethanes. J. Appl. Polym. Sci. 2011, 121, 1951–1956. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, J. A study on the performance of intumescent flame-retarded polypropylene with nano-ZrO2. J. Fire Sci. 2011, 29, 227–242. [Google Scholar] [CrossRef]
- Yew, M.; Yew, M.; Saw, L.; Ng, T.; Durairaj, R.; Beh, J. Influences of nano bio-filler on the fire-resistive and mechanical properties of water-based intumescent coatings. Prog. Org. Coat. 2018, 124, 33–40. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Zhang, X.; Khan, F.; Fahad, S.; Ullah, A. Adhesive properties of bio-based epoxy resin reinforced by cellulose nanocrystal additives. J. Polym. Eng. 2020, 40, 314–320. [Google Scholar] [CrossRef]
- Jamil, M.; Zhan, X.; Chen, F.; Cheng, D.; Zhang, Q. Durable and scalable candle soot icephobic coating with nucleation and fracture mechanism. Appl. Mater. Interfaces 2019, 11, 31532–31542. [Google Scholar] [CrossRef]
- Zhan, W.; Chen, L.; Gu, Z.; Jiang, J. Influence of graphene on fire protection of intumescent fire retardant coating for steel structure. Energy Rep. 2020, 6 (Suppl. S2), 693–697. [Google Scholar] [CrossRef]
- Beheshti, A.; Heris, S. Is MWCNT a good synergistic candidate in app–per–mel intumescent coating for steel structure? Prog. Org. Coat. 2016, 90, 252–257. [Google Scholar] [CrossRef]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Transparent epoxy coatings with improved electrical, barrier and thermal features made of mechanically dispersed carbon nanotubes. Prog. Org. Coat. 2017, 111, 196–201. [Google Scholar] [CrossRef]
- Karel, M.; Nývlt, J.; Chianese, A. Crystallization of pentaerythritol I. solubility, density and metastable zone width. Collect. Czechoslov. Chem. Commun. 1994, 59, 1261–1269. [Google Scholar] [CrossRef]
- Kazarinov, R.; Kowalczyk, K.; Łopiński, J.; Schmidt, B.; Rokicka, J. An intumescent coating system modified with waste poly(ethylene terephthalate) as a substitute for dipentaerythritol. Prog. Org. Coat. 2018, 125, 481–488. [Google Scholar] [CrossRef]
- Le Bras, M.; Bourbigot, S. Comprehensive study of the degradation of an intumescent EVA-based material during combustion. J. Mater. Sci. 1999, 34, 5777–5782. [Google Scholar] [CrossRef]
- Mariappan, T.; Agarwal, A. Influence of titanium dioxide on the thermal insulation of waterborne intumescent fire protective paints to structural steel. Prog. Org. Coat. 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J. Influences of expandable graphite modified by polyethylene glycol on fire protection of waterborne intumescent fire resistive coating. Surf. Coat. Technol. 2010, 204, 3599–3605. [Google Scholar] [CrossRef]










| Coating Symbol/Component (wt. Part) | R-0 | R-P1 | R-P2 | R-D1 | R-D2 | R-D3 |
|---|---|---|---|---|---|---|
| PVAc | 14.4 | 14.4 | 14.4 | 14.4 | 14.4 | 14.4 |
| APP | 39.0 | 39.0 | 39.0 | 39.0 | 39.0 | 39.0 |
| MEL | 19.5 | 19.5 | 19.5 | 19.5 | 19.5 | 19.5 |
| PER | 13.0 | 13.0 | 13.0 | 0 | 0 | 0 |
| CNTs | 0 | 1.0 1 | 2.0 2 | 0 | 0 | 0 |
| PER-CNTs | 0 | 0 | 0 | 14.0 3 | 15.0 4 | 16.0 5 |
| TiO2 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| ZB | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Additives | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 |
| Sample Symbol | CNTs Dose 1 | Paint Viscosity (Pa.s) | Thermal Insulation Time, TIT (Min) | Intumescent Factor, IF (a.u.) | Weight Loss Temperature 2 (°C) | Calcination Residue 3 (wt.%) | ||
|---|---|---|---|---|---|---|---|---|
| T5 | T30 | T50 | ||||||
| R-0 | 0 | 0.59 | 25.4 | 9.9 ± 1.1 | 261 | 366 | 464 | 26.5 |
| R-P1 | 1 4 | 0.78 | 19.6 | 4.4 ± 1.1 | 262 | 375 | 477 | 25.4 |
| R-P2 | 2 4 | 0.97 | 12.9 | 1.3 ± 0.2 | 255 | 366 | 522 | 28.1 |
| R-D1 | 1 5 | 0.60 | 28.7 | 17.6 ± 2.7 | 266 | 373 | 482 | 26.9 |
| R-D2 | 2 5 | 0.59 | 30.0 | 17.9 ± 1.4 | 259 | 370 | 465 | 25.9 |
| R-D3 | 3 5 | 0.61 | 28.8 | 20.0 ± 1.0 | 249 | 361 | 466 | 27.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, M.; Łopiński, J.; Kowalczyk, A.; Kowalczyk, K. Thermoplastic Intumescent Coatings Modified with Pentaerythritol-Occluded Carbon Nanotubes. Materials 2021, 14, 6284. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14216284
Tomczak M, Łopiński J, Kowalczyk A, Kowalczyk K. Thermoplastic Intumescent Coatings Modified with Pentaerythritol-Occluded Carbon Nanotubes. Materials. 2021; 14(21):6284. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14216284
Chicago/Turabian StyleTomczak, Michał, Jakub Łopiński, Agnieszka Kowalczyk, and Krzysztof Kowalczyk. 2021. "Thermoplastic Intumescent Coatings Modified with Pentaerythritol-Occluded Carbon Nanotubes" Materials 14, no. 21: 6284. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14216284