Combining Fiber Enzymatic Pretreatments and Coupling Agents to Improve Physical and Mechanical Properties of Hemp Hurd/Wood/Polypropylene Composite
Abstract
:1. Introduction
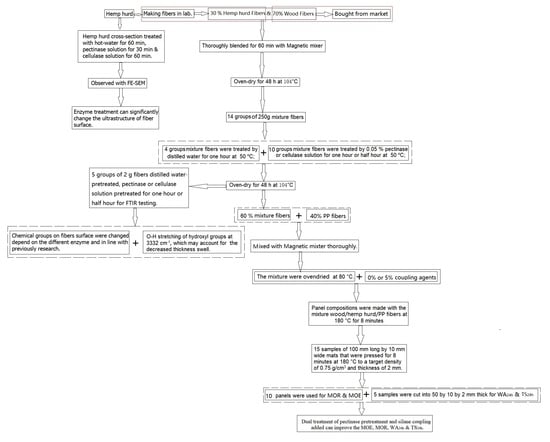
2. Materials and Methods
2.1. Fiber Preparation
2.2. Enzymatic Pretreatments
2.3. Panel Manufacturing
2.4. Water Uptake Properties
2.5. Bending Properties
2.6. Fiber Characterization
3. Results and Discussion
3.1. Effects of Fiber Pretreatment on Panel Moisture Behaviour
3.1.1. Thickness Swelling for 24 h (TS24h)
3.1.2. Water Absorption for 24 h (WA24h)
3.2. Effects of Fiber Pretreatment on Panel Mechanical Properties
3.2.1. Effect of Pretreatment on Elastic Modulus of Composites (MOE)
3.2.2. Effect of Fiber Pretreatment on MOR
3.3. FTIR Analysis
3.4. Effect of Fiber Pretreatment on Ultrastructure Hemp Hurd
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemons, C. Wood-plastic composites in the United States: The interfacing of two industries. For. Prod. J. 2002, 52, 10–18. [Google Scholar]
- Huang, R.; Mei, C.; Xu, X.; Kärki, T.; Lee, S.; Wu, Q. Effect of Hybrid Talc-Basalt Fillers in the Shell Layer on Thermal and Mechanical Performance of Co-Extruded Wood Plastic Composites. Materials 2015, 8, 8510–8523. [Google Scholar] [CrossRef] [Green Version]
- Gallos, A.; Paës, G.; Allais, F.; Beaugrand, J. Lignocellulosic fibers: A critical review of the extrusion process for enhancement of the properties of natural fiber composites. RSC Adv. 2017, 7, 34638–34654. [Google Scholar] [CrossRef]
- Mankowski, M.; Morrell, J.J. Patterns of fungal attack in wood-plastic composites following exposure in a soil block test. Wood Fiber Sci. 2000, 32, 340–345. [Google Scholar]
- Morrell, J.J.; Stark, N.M.; Pendleton, D.E.; McDonald, A.G. Durability of wood-plastic composites. Wood Des. Focus 2006, 16, 7–10. [Google Scholar]
- Martins, G.; Antunes, F.; Mateus, A.; Malça, C. Optimization of a Wood Plastic Composite for Architectural Applications. Procedia Manuf. 2017, 12, 203–220. [Google Scholar] [CrossRef]
- Tanas, F.; Znoag, M.; Teac, C.; Nechifor, M.; Shahzad, A. Modified hemp fibers intended for fiber-reinforced polymer com-posites used in structural applications—A review. I. methods of modification. Polym. Compos. 2020, 41, 5–31. [Google Scholar] [CrossRef]
- Li, Y.; Pickering, K.L. Hemp fiber reinforced composites using chelator and enzyme treatments. Compos. Sci. Technol. 2008, 68, 3293–3298. [Google Scholar] [CrossRef]
- Tran-Le, A.D.; Nguyen, S.-T.; Langlet, T. A novel anisotropic analytical model for effective thermal conductivity tensor of dry lime-hemp concrete with preferred spatial distributions. Energy Build. 2019, 182, 75–87. [Google Scholar] [CrossRef]
- Xiao, X.; Chevalia, S.V.; Song, P.; He, D.; Wang, H. Polylactide/hemp hurd biocomposites as sustainable 3d printing feed-stock. Compos. Sci. Technol. 2019, 184, 107887. [Google Scholar] [CrossRef]
- Yashas Gowda, T.G.; Sanjay, M.R.; Subrahmanya, K.; Bhat Madhu, P.; Senthamaraikannan, P.; Yogesha, B. Polymer matrix-natural fiber composites: An overview. Cogent Eng. 2018, 5, 1446667. [Google Scholar] [CrossRef]
- Bouafif, H.; Koubaa, A.; Perré, P.; Cloutier, A. Effects of fiber characteristics on the physical and mechanical properties of wood plastic composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1975–1981. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Espinach, F.X.; Pelach, M.N.; Méndez, J.A.; Vilaseca, F.; Tarrés, Q. Effect of NaOH treatment on the flexural mod-ulus of hemp core reinforced composites and on the intrinsic flexural moduli of the fibers. Polymers 2020, 12, 1428–1448. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Z.; Wu, Q.; McNabb, H.S. Chemical coupling in wood fiber and polymer composites: A review of coupling agents and treatments. Wood Fiber Sci. 2007, 32, 88–104. [Google Scholar]
- Meon, M.S.; Othman, M.F.; Husain, H.; Remeli, M.F.; Syawal, M.S.M. Improving Tensile Properties of Kenaf Fibers Treated with Sodium Hydroxide. Procedia Eng. 2012, 41, 1587–1592. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zhang, Y.; Wang, S. Preparation and characterization of acrylonitrile-butadiene-styrene nanocomposites reinforced with cellulose nanocrystal via solution casting method. Polym. Compos. 2015, 38, 167–173. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: A review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Mellerowicz, E.J.; Sundberg, B. Wood cell walls: Biosynthesis, development dynamics and their implications for wood properties. Curr. Opin. Plant Biol. 2008, 11, 293–300. [Google Scholar] [CrossRef]
- Mamun, A.A.; Bledzki, A.K. Micro fiber reinforced PLA and PP composites: Enzyme modification, mechanical and thermal properties. Compos. Sci. Technol. 2013, 165, 1912–1920. [Google Scholar]
- Palin, R.; Geitmann, A. The role of pectin in plant morphogenesis. Biosystems 2012, 109, 397–402. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G.; Freitag, C.; Morrell, J.J. Effects of wood fiber esterification on properties, weatherability, and biodura-bility of wood plastic composites. Polym. Degrad. Stab. 2013, 98, 1348–1361. [Google Scholar] [CrossRef]
- Cosgrove, J.D. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Mussone, P.G.; Alemaskin, K.; Chae, M.; Wolodko, J.; Bressler, D.C. Enzymatically treated natural fibers as rein-forcing agents for biocomposite material: Mechanical, thermal, and moisture absorption characterization. J. Mater. Sci. 2016, 51, 2677–2686. [Google Scholar] [CrossRef]
- Zhang, Q. Research Progress of new wood-plastic composite material. New Chem. Mater. 2014, 42, 6–8. [Google Scholar]
- Ge, Z.; Si, D.; Zhang, S. Press forming and properties of foamed wood plastic composite material of polyethylene/straw flour. Plastic 2015, 44, 115–118. [Google Scholar]
- Yang, M.; Morrell, J.J.; Li, X.; Liu, Y. Effect of different fiber ratios and pectin pretreatment on mechanical properties of wood-plastic composites. Wood Process. Mach. 2019, 30, 16–19. [Google Scholar]
- European Union. Standard EN317, Particleboards and Fibreboards–Determination of Swelling in Thickness after Immersion in Water; European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- ASTM. Standard D790-02. Standard test methods for flexural properties of unreinforced and reinforced plastics and electrical insulating materials. In ASTM Annual Book of Standards Volume 4.10 Wood; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Naumann, A.; Gonzales, M.N.; Peddireddi, S.; Kues, U.; Polle, A. Fourier transform infrared microscopy and imaging: Detection of fungi in wood. Fungal Genet. Biol. 2005, 42, 829–835. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Pandey, K.; Pitman, A. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Xie, Q.; Zeng, H.; Peng, Q.; Bressy, C.; Ma, C.; Zhang, G. Self-stratifying silicone coating with nonleaching antifoulant for ma-rine anti-biofouling. Adv. Mater. Interfaces 2019, 6, 1900535. [Google Scholar] [CrossRef]
- Wang, W.; Morrell, J.J. Water sorption characteristics of two wood-plastic composites. For. Prod. J. 2004, 54, 209–212. [Google Scholar]
- Li, X.; Cappellazzi, J.; Morrell, J.J. Effect of particle pre-treatments on the quality of kenaf core/HDPE plastic composites. Bioresour. 2020, 15, 6262–6272. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Li, X.; Xiao, R.; Morrell, J.J.; Zhou, X.; Du, G. Improving the performance of hemp hurd/polypropylene composites using pec-tinase pre-treatments. Ind. Crop. Prod. 2017, 97, 465–468. [Google Scholar] [CrossRef]









| Pretreatment | Coupling Agent (%) | Samples’ Name | |||
|---|---|---|---|---|---|
| Enzyme | Time (min) | Silane | Titanate | Maleic Anhydride | |
| none | - | - | - | - | Control |
| - | 5.0 | - | - | Silane | |
| - | - | 5.0 | - | Titanate | |
| - | - | - | 5.0 | Maleic anhydride | |
| 0.05% pectinase | 60 | - | - | - | P + 60 |
| 30 | - | - | - | P + 30 | |
| 30 | 5.0 | - | - | P + 30 + Silane | |
| 30 | - | 5.0 | - | P + 30 + Titanate | |
| 30 | 2.5 | 2.5 | - | P + 30 + Silane/Titanate | |
| 0.05% cellulase | 30 | - | - | - | C + 30 |
| 60 | - | - | - | C + 60 | |
| 60 | 5.0 | - | - | C + 60 + Silane | |
| 60 | - | 5.0 | - | C + 60 + Titanate | |
| 60 | 2.5 | 2.5 | - | C + 60 + Silane/Titanate | |
| Wave Number (cm−1) | Band Assignment | References |
|---|---|---|
| 3332 | O-H stretching of bonded hydroxyl groups | [29,30,31] |
| 2896 | Symmetric CH stretching in aromatic methoxyl groups and in methyl and methylene groups of side chains | [30,31] |
| 1732 | C=O stretching in xylans (unconjugated) | [30,31,32] |
| 1635 | H-O-H deformation vibration of absorbed water and C=O stretching in lignin | [30,32] |
| 1592 | C=C stretching of the aromatic ring (S)Aromatic skeletal vibrations + C=O stretching S ≥ G | [30,31,32] |
| 1504 | C=C stretching of the aromatic ring (G)Aromatic skeletal vibrations in lignin | [30,31,32] |
| 1452 | CH2 deformation vibrations in lignin and xylans | [30,31] |
| 1421 | C–H asymmetric deformation in –OCH3Aromatic skeletal vibrations combined with C-Hin plane deformation + C-H deformation in lignin and carbohydrates | [29,31,33] |
| 1367 | C-H deformation in cellulose and hemicelluloses | [29,30,31] |
| 1318 | C-H vibration in cellulose + C1-O vibration insyringyl derivatives | [30,31] |
| 1233 | Acetyl and carboxyl vibrations in xylans and C=O stretching vibrations in lignin | [30,31] |
| 1155 | C-O-C vibration in cellulose and hemicelluloses | [30,31] |
| 1097 | Aromatic C–H in-plane deformation (typical for S units), C=O stretch O-H association band in cellulose and hemicelluloses | [30,31] |
| 1029 | C=O stretching vibration in cellulose, hemicelluloses and lignin | [30,31] |
| 895 | C-H deformation in cellulose | [29,30,31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Qiang, M.; Yang, M.; Morrell, J.J.; Zhang, N. Combining Fiber Enzymatic Pretreatments and Coupling Agents to Improve Physical and Mechanical Properties of Hemp Hurd/Wood/Polypropylene Composite. Materials 2021, 14, 6384. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14216384
Li X, Qiang M, Yang M, Morrell JJ, Zhang N. Combining Fiber Enzymatic Pretreatments and Coupling Agents to Improve Physical and Mechanical Properties of Hemp Hurd/Wood/Polypropylene Composite. Materials. 2021; 14(21):6384. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14216384
Chicago/Turabian StyleLi, Xiaoping, Mingli Qiang, Mingwei Yang, Jeffrey J. Morrell, and Neng Zhang. 2021. "Combining Fiber Enzymatic Pretreatments and Coupling Agents to Improve Physical and Mechanical Properties of Hemp Hurd/Wood/Polypropylene Composite" Materials 14, no. 21: 6384. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14216384