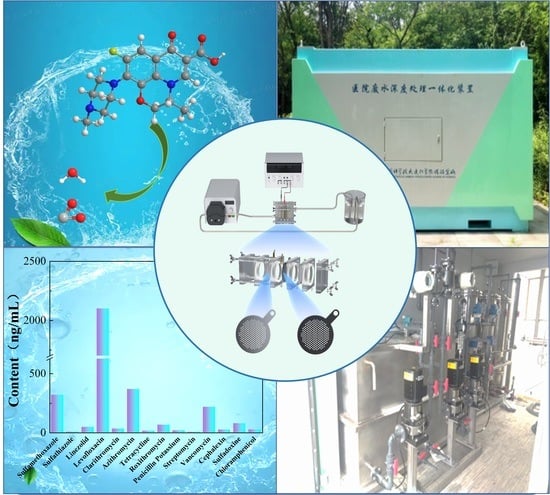
Electrocatalytic Degradation of Levofloxacin, a Typical Antibiotic in Hospital Wastewater
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Experimental Instrument
2.3. Electrode Preparation
2.4. TOC and LVX Content Analysis
2.5. Experimental Design
2.6. Electrooxidation Experiment
2.7. Model Fitting and Data Analysis
2.8. Fluorescence Measurement
3. Results and Discussion
3.1. Stability Assessment
3.1.1. Scanning Electron Microscope (SEM) Analysis
3.1.2. Atomic Force Microscopy (AFM) Analysis
3.1.3. X-ray Diffraction (XRD) Analysis
3.1.4. XPS Analysis
3.2. Electrochemical Analysis
3.3. ANOVA and Model Simplification
3.4. Response Surface Single Factor Investigation
3.5. Response Surface Multifactor Interaction
3.6. Experimental Verification
3.7. Exploration of Degradation Mechanism
3.8. Possible Degradation Routes of LVX
3.9. Treatment of Wastewater from Novel Coronavirus Epidemic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, K.; Agrawal, K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- You, X.; Wu, D.; Wei, H.; Xie, B.; Lu, J. Fluoroquinolones and beta-lactam antibiotics and antibiotic resistance genes in autumn leachates of seven major municipal solid waste landfills in China. Environ. Int. 2018, 113, 162–169. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.-H.; Wang, Z.; Liu, C.-X.; Huang, X.; Zhu, G. Behavior of tetracycline and sulfamethazine with corresponding resistance genes from swine wastewater in pilot-scale constructed wetland. J. Hazard. Mater. 2014, 278, 304–310. [Google Scholar] [CrossRef]
- Ge, L.; Na, G.; Zhang, S.; Li, K.; Zhang, P.; Ren, H.; Yao, Z. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity change. Sci. Total Environ. 2015, 527–528, 12–17. [Google Scholar] [CrossRef]
- Sinha, K.; Biswas, P. Structural elucidation of Levofloxacin and Ciprofloxacin using density functional theory and Raman spectroscopy with inexpensive lab-built setup. J. Mol. Struct. 2020, 1222, 128946. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Ge, R.; Yang, S.; Zhai, Y.; Hua, T.; Ondon, B.S.; Zhou, Q.; Li, F. Microbial electro-Fenton: A promising system for antibiotics resistance genes degradation and energy generation. Sci. Total Environ. 2020, 699, 134160. [Google Scholar] [CrossRef] [PubMed]
- Perini, L.; Tonetti, L.; Vidal, C.; Montagner, C.; Nogueira, P. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation. Appl. Catal. B 2018, 224, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Verma, N. Evaluation of degradation and mineralization of glyphosate pollutant in wastewater using catalytic wet air oxidation over Fe-dispersed carbon nanofibrous bead. Chem. Eng. J. 2021, 417, 128029. [Google Scholar] [CrossRef]
- Segura, Y.; Cruz, A.; Munoz, M.; Alvarez, S.; Garcia, J.; Casas, A.; De Pedro, Z.M.; Martinez, F. A comparative study among catalytic wet air oxidation, Fenton, and Photo-Fenton technologies for the on-site treatment of hospital wastewater. J. Environ. Manag. 2021, 290, 112624. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Chen, Z.; Yang, X.; Zhao, W.; Liu, C.; Sun, T.; Zhou, D.; Yang, Q.; Wei, G.; Fan, M. Perovskite cesium lead bromide quantum dots: A new efficient photocatalyst for degrading antibiotic residues in organic system. J. Clean. Prod. 2020, 249, 119335. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L. Enhanced photocatalytic oxidation degradability for real cyanide wastewater by designing photocatalyst GO/TiO2/ZSM-5: Performance and mechanism research. Chem. Eng. J. 2022, 428, 131257. [Google Scholar] [CrossRef]
- Eshagh, S.; Hassaninejad, S. Synergistic effects of nanozeolite beta-MWCNTs on the electrocatalytic oxidation of ethylene glycol: Experimental design by response surface methodology. Mater. Sci. Eng. B Adv. 2021, 268, 115125. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, S.; Sun, W.; Zheng, H. Degradation of high-chemical oxygen demand concentration pesticide wastewater by 3D electrocatalytic oxidation. Chin. J. Chem. Eng. 2019, 7, 103276. [Google Scholar] [CrossRef]
- Teng, J.; You, S.; Ma, F.; Chen, X.; Ren, N. Enhanced electrochemical decontamination and water permeation of titanium suboxide reactive electrochemical membrane based on sonoelectrochemistry. Ultrason. Sonochem. 2020, 69, 105248. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y. Spinel CuxCo1−xMn2O4 electrode for effectively cleaning organic wastewater via electrocatalytic oxidation. Sep. Purif. Technol. 2021, 258, 118024. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Han, B. Electroreduction of CO2 in Ionic Liquid-Based Electrolyte. Innovation 2020, 1, 100016. [Google Scholar] [CrossRef]
- Yuan, Y.; Adimi, S.; Thomas, T.; Wang, J.; Guo, H.; Chen, J.; Attfield, J.P.; DiSalvo, F.J.; Yang, M. Co3Mo3N—An efficient multifunctional electrocatalyst. Innovation 2021, 2, 100096. [Google Scholar]
- Ding, J.; Bu, L.; Zhao, Q.; Kabutey, T.; Wei, L.; Dionysiou, D. Electrochemical activation of persulfate on BDD and DSA anodes: Electrolyte influence, kinetics and mechanisms in the degradation of bisphenol A. J. Hazard. Mater. 2020, 388, 121789. [Google Scholar] [CrossRef]
- Murugananthan, M.; Yoshihara, S.; Rakuma, T.; Uehara, N.; Shirakashi, T. Electrochemical degradation of 17β-estradiol (E2) at boron-doped diamond (Si/BDD) thin film electrode. Electrochim. Acta 2007, 52, 3242–3249. [Google Scholar] [CrossRef]
- Huang, K.; Chao, P.; Kuo, Y.; Chi, K.; Cheng, M.; Wu, Z.; Huang, T.Y. Response surface methodology-based fabrication of boron-doped diamond electrodes for electrochemical degradation of guaifenesin in aqueous solution. J. Taiwan Inst. Chem. E 2021, 123, 124–133. [Google Scholar] [CrossRef]
- Shi, L.; Xu, F.; Gao, J.; Yuen, M.; Sun, S.; Xu, J.; Jia, K.; Zuo, D. Nanostructured boron-doped diamond electrode for degradation of the simulation wastewater of phenol. Diam. Relat. Mater. 2020, 109, 108098. [Google Scholar] [CrossRef]
- Marks, H.; Kerpen, K.; Diesing, D.; Telgheder, U. Electrochemical degradation of perfluorooctanoic acid in aqueous solution by boron-doped diamond electrodes under pulsed voltage condition. J. Electroanal. Chem. 2021, 895, 115415. [Google Scholar] [CrossRef]
- Pei, S.; Shi, H.; Zhang, J.; Wang, S.; Ren, N.; You, S. Electrochemical removal of tetrabromobisphenol A by fluorine-doped titanium suboxide electrochemically reactive membrane. J. Hazard. Mater. 2021, 419, 126434. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Liu, G.; Liang, J.; You, S. Electrochemical oxidation of sulfadiazine with titanium suboxide mesh anode. Electrochim. Acta 2020, 331, 135441. [Google Scholar] [CrossRef]
- Yang, X.; Jiuji, G.; Zhaowu, Z.; Zhang, H.; Qi, T. Doping effects on the electro-degradation of phenol on doped titanium suboxide anode. Chin. J. Chem. Eng. 2018, 26, 830–837. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, H.; Teng, J.; You, S. Electrochemical degradation of perfluorooctanoic acid by macro-porous titanium suboxide anode in the presence of sulfate. Chem. Eng. J. 2019, 371, 7–14. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Chen, Q.; Zhou, J. Coupling effect of nitrogen-doped carbon black and carbon nanotube in assembly gas diffusion electrode for H2O2 electro-generation and recalcitrant pollutant degradation. Sep. Purif. Technol. 2021, 265, 118493. [Google Scholar] [CrossRef]
- Duan, P.; Chen, D.; Hu, X. Tin dioxide decorated on Ni-encapsulated nitrogen-doped carbon nanotubes for anodic electrolysis and persulfate activation to degrade cephalexin: Mineralization and degradation pathway. Chemosphere 2020, 269, 128740. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, X.; Yang, Z.; Liu, Y.; Zhou, Z.; Ren, Z. Investigating the influences of electrode material property on degradation behavior of organic wastewaters by iron-carbon micro-electrolysis. Chem. Eng. J. 2018, 338, 46–54. [Google Scholar] [CrossRef]
- Zhen, W.; Xuanqi, K.; Shangyuan, X.; Xiaokang, Z.; Bo, J.; Qing, F. Electrochemical oxidation of Rhodamine B with cerium and sodium dodecyl benzene sulfonate co-modified Ti/PbO2 electrodes: Preparation, characterization, optimization, application. Chin. J. Chem. Eng. 2020, 32, 191–202. [Google Scholar]
- Deng, D.; Wu, X.; Li, M.; Qian, S.; Tang, B.; Wei, S.; Zhang, J. Electrochemical degradation of three phthalate esters in synthetic wastewater by using a Ce-doped Ti/PbO2 electrode. Chemosphere 2020, 259, 127488. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Sepúlveda, P.; García, A.; Martins, D.; Salazar, R. Degradation of oxamic acid using dimensionally stable anodes (DSA) based on a mixture of RuO2 and IrO2 nanoparticle. Chemosphere 2020, 251, 126674. [Google Scholar] [CrossRef] [PubMed]
- Krstić, V.; Pešovski, B. Reviews the research on some dimensionally stable anodes (DSA) based on titanium. Hydrometallurgy 2019, 185, 71–75. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Zhou, J.; Shen, Q.; Cao, L.; Yang, J. Electrochemical removal of gaseous elemental mercury in liquid phase with a novel foam titanium-based DSA anode. Sep. Purif. Technol. 2020, 250, 117162. [Google Scholar] [CrossRef]
- Abdalrhman, S.; Gamal, M. Degradation of organics in real oil sands process water by electro-oxidation using graphite and dimensionally stable anode. Chem. Eng. J. 2020, 389, 124406. [Google Scholar] [CrossRef]
- Pekmez, N.; Uğur, M.; Karaca, E.; Ertekin, Z.; Pekmez, K. Room temperature electrosynthesis of TinO2n-1 film and its bilayer with PNMPy on mild steel for corrosion protection in sulphuric acid. Electrochim. Acta 2021, 376, 137996. [Google Scholar] [CrossRef]
- Du, J.; Zhao, J.; Ren, J. Interface effect of C3N4-Ti4O7-MoS2 composite toward enhanced electrocatalytic hydrogen evolution reaction. J. Fuel Chem. Technol. 2021, 49, 986–996. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, Y.; Shang, K.; Lu, N.; Li, J.; Wu, Y. Degradation of toluene by pulse-modulated multistage DBD plasma: Key parameters optimization through response surface methodology (RSM) and degradation pathway analysis. J. Hazard. Mater. 2020, 393, 122365. [Google Scholar] [CrossRef]
- Toor, A.; Duong, T.; Ko, S.; Hussain, F.; Oh, E. Optimization of Fenton process for removing TOC and color from swine wastewater using response surface method (RSM). J. Environ. Manag. 2021, 279, 111625. [Google Scholar] [CrossRef]
- Okey, F.; Chukwuma, E.; Okoye, C.; Onukwuli, D. Response Surface Methodology optimization of chito-protein synthesized from crab shell in treatment of abattoir wastewater. Heliyon 2020, 6, e05186. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhou, D.; Zhang, Q. Synthesis and characterization of Magneli phases: Reduction of TiO2 in a decomposed NH3 atmosphere. Mater. Lett. 2012, 79, 42–44. [Google Scholar] [CrossRef]
- Perea, A.; Palma-Goyes, E.; Vazquez, J.; Romero, I.; Ostos, C.; Torres, A. Efficient cephalexin degradation using active chlorine produced on ruthenium and iridium oxide anodes: Role of bath composition, analysis of degradation pathways and degradation extent. Sci. Total Environ. 2019, 648, 377–387. [Google Scholar] [CrossRef]
- Ma, L.; Lv, H.; Yu, H.; Kong, L.; Zhang, R.; Guo, X.; Jin, H.; He, G.; Liu, X. In-depth investigation on the factors affecting the performance of high oil-absorption resin by response surface method. Chin. J. Chem. Eng. 2021, 332, 86–96. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Shi, S.; Liu, Y. Microwave enhanced Fenton-like process for the treatment of high concentration pharmaceutical wastewater. J. Hazard. Mater. 2009, 168, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.; Boaventura, R.; Brillas, E.; Vilar, P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewater. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Di, P.; Yue, L.; Song, H.; Chen, D.; Zhu, W.; Liu, R.; Yang, H.; Li, A.; Zhang, S. Trace Ti3+ and N-codoped TiO2 nanotube array anode for significantly enhanced electrocatalytic degradation of tetracycline and metronidazole. Chem. Eng. J. 2021, 405, 126982. [Google Scholar]
- Li, S.; Wang, C.; Cai, M.; Yang, F.; Liu, Y.; Chen, J.; Zhang, P.; Li, X.; Chen, X. Facile fabrication of TaON/Bi2MoO6 core–shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J. 2022, 428, 131158. [Google Scholar] [CrossRef]
- Yang, X.; Xie, X.; Li, S.; Zhang, W.; Zhang, X.; Chai, H.; Huang, Y. The POM@MOF hybrid derived hierarchical hollow Mo/Co bimetal oxides nanocages for efficiently activating peroxymonosulfate to degrade levofloxacin. J. Hazard. Mater. 2021, 419, 126360. [Google Scholar] [CrossRef]
- Meng, F.; Wang, Y.; Chen, Z.; Hu, J.; Lu, G.; Ma, W. Synthesis of CQDs@FeOOH nanoneedles with abundant active edges for efficient electro-catalytic degradation of levofloxacin: Degradation mechanism and toxicity assessment. Appl. Catal. B 2021, 282, 119597. [Google Scholar] [CrossRef]
- Prabavathi, L.; Saravanakumar, K.; Park, M.; Muthuraj, V. Photocatalytic degradation of levofloxacin by a novel Sm6WO12/g-C3N4 heterojunction: Performance, mechanism and degradation pathway. Sep. Purif. Technol. 2021, 257, 117985. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Wang, M.; Jin, C.; Kang, J.; Tang, Y.; Li, S. Eu2O3/Co3O4 nanosheets for levofloxacin removal via peroxymonosulfate activation: Performance, mechanism and degradation pathway. Sep. Purif. Technol. 2021, 274, 118666. [Google Scholar] [CrossRef]
- Shen, J.; Qian, L.; Huang, J.; Guo, Y.; Zhang, Z. Enhanced degradation toward Levofloxacin under visible light with S-scheme heterojunction In2O3/Ag2CO3: Internal electric field, DFT calculation and degradation mechanism. Sep. Purif. Technol. 2021, 275, 119239. [Google Scholar] [CrossRef]
- Zhong, Y.; Shih, K.; Diao, Z.; Song, G.; Su, M.; Hou, L.; Chen, D.; Kong, L. Peroxymonosulfate activation through LED-induced ZnFe2O4 for levofloxacin degradation. Chem. Eng. J. 2021, 417, 129225. [Google Scholar] [CrossRef]
- Goulart, L.; Moratalla, A.; Lanza, M.; Saez, C.; Rodrigo, M. Photocatalytic performance of Ti/MMO/ZnO at degradation of levofloxacin: Effect of pH and chloride anion. J. Electroanal. Chem. 2021, 880, 114894. [Google Scholar] [CrossRef]
- Lyu, J.; Ge, M.; Hu, Z.; Guo, C. One-pot synthesis of magnetic CuO/Fe2O3/CuFe2O4 nanocomposite to activate persulfate for levofloxacin removal: Investigation of efficiency, mechanism and degradation rout. Chem. Eng. J. 2020, 389, 124456. [Google Scholar] [CrossRef]











| Factor Name | Number | Unit | Coded Value | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |||
| Initial pH value | A | 0.55 | 4 | 6.5 | 9 | 12.4 | |
| Current density | B | A/m2 | 5.6 | 17 | 28.3 | 39.6 | 51.0 |
| Flow rate | C | mL/min | 5.20 | 50 | 82.5 | 115 | 159.7 |
| Reaction time | D | min | 18.6 | 60 | 90 | 120 | 161.3 |
| Chloride ion content | E | ‰ | 0.62 | 2 | 3 | 4 | 5.38 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 3.10 × 10−1 | 19 | 1.63 × 10−2 | 6.95 | <0.0001 | significant |
| A-pH | 6.36 × 10−4 | 1 | 6.36 × 10−4 | 2.71 × 10−1 | 0.6067 | - |
| B-current density | 5.11 × 10−2 | 1 | 5.11 × 10−2 | 21.76 | <0.0001 | - |
| C-flow rate | 5.73 × 10−6 | 1 | 5.73 × 10−6 | 2.44 × 10−3 | 0.9609 | - |
| D-reaction time | 7.72 × 10−2 | 1 | 7.72 × 10−2 | 32.89 | <0.0001 | - |
| E-chloride ion concentration | 2.03 × 10−3 | 1 | 2.03 × 10−3 | 8.64 × 10−1 | 0.3601 | - |
| AB | 2.31 × 10−4 | 1 | 2.31 × 10−4 | 9.85 × 10−2 | 0.7558 | - |
| AC | 1.04 × 10−5 | 1 | 1.04 × 10−5 | 4.43 × 10−3 | 0.9473 | - |
| AD | 1.60 × 10−3 | 1 | 1.60 × 10−3 | 6.82 × 10−1 | 0.4154 | - |
| AE | 2.17 × 10−2 | 1 | 2.17 × 10−2 | 9.25 | 0.0049 | - |
| BC | 2.69 × 10−3 | 1 | 2.69 × 10−3 | 1.14 | 0.2932 | - |
| BD | 7.86 × 10−5 | 1 | 7.86 × 10−5 | 3.35 × 10−2 | 0.8561 | - |
| DE | 7.00 × 10−4 | 1 | 7.00 × 10−4 | 2.98 × 10−1 | 0.5890 | - |
| A2 | 5.31 × 10−2 | 1 | 5.31 × 10−2 | 22.63 | <0.0001 | - |
| B2 | 4.29 × 10−5 | 1 | 4.29 × 10−5 | 1.83 × 10−2 | 0.8934 | - |
| ABC | 6.21 × 10−3 | 1 | 6.21 × 10−3 | 2.64 | 0.1145 | - |
| ABD | 7.57 × 10−3 | 1 | 7.57 × 10−3 | 3.22 | 0.0827 | - |
| ADE | 7.63 × 10−3 | 1 | 7.63 × 10−3 | 3.25 | 0.0816 | - |
| A2B | 1.48 × 10−2 | 1 | 1.48 × 10−2 | 6.30 | 0.0177 | - |
| AB2 | 1.00 × 10−2 | 1 | 1.00 × 10−2 | 4.26 | 0.0478 | - |
| Residual | 7.04 × 10−2 | 30 | 2.35 × 10−3 | - | - | - |
| Lack of Fit | 6.83 × 10−2 | 23 | 2.97 × 10−3 | 9.77 | 0.0024 | significant |
| Pure error | 2.13 × 10−3 | 7 | 3.04 × 10−4 | - | - | - |
| Cor total | 0.38 | 49 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Han, P.; Li, X.; Mu, Z.; Zuo, Y.; Wang, X.; Tan, Y.; He, G.; Jin, H.; Sun, C.; et al. Electrocatalytic Degradation of Levofloxacin, a Typical Antibiotic in Hospital Wastewater. Materials 2021, 14, 6814. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14226814
Lv H, Han P, Li X, Mu Z, Zuo Y, Wang X, Tan Y, He G, Jin H, Sun C, et al. Electrocatalytic Degradation of Levofloxacin, a Typical Antibiotic in Hospital Wastewater. Materials. 2021; 14(22):6814. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14226814
Chicago/Turabian StyleLv, Hongxia, Peiwei Han, Xiaogang Li, Zhao Mu, Yuan Zuo, Xu Wang, Yannan Tan, Guangxiang He, Haibo Jin, Chenglin Sun, and et al. 2021. "Electrocatalytic Degradation of Levofloxacin, a Typical Antibiotic in Hospital Wastewater" Materials 14, no. 22: 6814. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14226814