Stress-Dependent Particle Interactions of Magnesium Aluminometasilicates as Their Performance Factor in Powder Flow and Compaction Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Physical Powder Mixtures
2.3. Quantification of Particle Interaction
2.3.1. Analysis of Particle Interactions under Marginal or Mild Stress Condition
2.3.2. Analysis of Particle Interactions under Compaction Condition
2.4. Visual Observation
3. Results and Discussion
3.1. Powder Characterization
3.2. SEM/BSE Observation
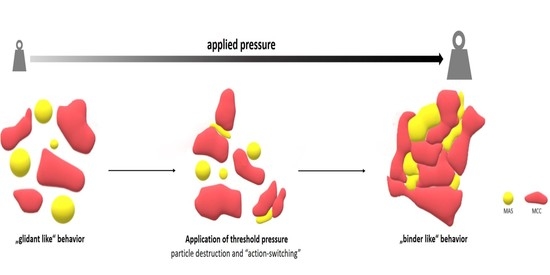
3.3. Effect of Applied Pressure on Particle Interaction Creation
3.3.1. Neusilin® US2
- Marginal or Mild Stress Condition
- Compaction Condition
3.3.2. Neusilin® S2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarmolińska, S.; Feliczak-Guzik, A.; Nowak, I. Synthesis, characterization and use of mesoporous silicas of the following types sba-1, sba-2, hmm-1 and hmm-2. Materials 2020, 13, 4385. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Cui, S.; Ma, X.; Guo, H.; Wang, Y. Preparation of cu-al/sio2 porous material and its effect on no decomposition in a cement kiln. Materials 2020, 13, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, K.; Santra, S.; Zhao, X.; Hilliard, L.R.; Smith, J.E.; Wu, Y.; Tan, W. Watching silica nanoparticles glow in the biological world. Anal. Chem. 2006, 78, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Razzaque, S.; Hussain, S.Z.; Hussain, I.; Tan, B. Design and utility of metal/metal oxide nanoparticles mediated by thioether end-functionalized polymeric ligands. Polymers 2016, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.E.; Amira, M.F.; Zaghloul, A.A.; Ibrahim, G.A.A. High performance microwave-enforced solid phase extraction of heavy metals from aqueous solutions using magnetic iron oxide nanoparticles-protected-nanosilica. Sep. Purif. Technol. 2016, 163, 169–172. [Google Scholar] [CrossRef]
- Huang, C.-L. A study of the optical properties and fabrication of coatings made of three-dimensional photonic glass. Coatings 2020, 10, 781. [Google Scholar] [CrossRef]
- Augsburger, L.L.; Shangraw, R.F. Effect of glidants in tableting. J. Pharm. Sci. 1966, 55, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Gold, G.; Duvall, R.N.; Palermo, B.T.; Slater, J.G. Powder flow studies ii: Effect of glidants on flow rate and angle of repose. J. Pharm. Sci. 1966, 55, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, I.; Eber, M.; Meyer, K. Nanomaterials as flow regulators in dry powders. Z. Phys. Chem. 2004, 218, 102–151. [Google Scholar] [CrossRef]
- Jonat, S.; Albers, P.; Gray, A.; Schmidt, P.C. Investigation of the glidant properties of compacted colloidal silicon dioxide by angle of repose and x-ray photoelectron spectroscopy. Eur. J. Pharm. Biopharm. 2006, 63, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.-K.; Ruppel, J.; Drexel, C.-P.; Zimmermann, I. Precipitated silica as flow regulator. Eur. J. Pharm. Sci. 2008, 34, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Zimmermann, I. Effect of glidants in binary powder mixtures. Powder Technol. 2004, 139, 40–54. [Google Scholar] [CrossRef]
- Rumpf, H. Die wissenschaft des agglomerierens. Chem. Ing. Tech. 1974, 46, 1–11. [Google Scholar] [CrossRef]
- Jonat, S.; Hasenzahl, S.; Drechsler, M.; Albers, P.; Wagner, K.G.; Schmidt, P.C. Investigation of compacted hydrophilic and hydrophobic colloidal silicon dioxides as glidants for pharmaceutical excipients. Powder Technol. 2004, 141, 31–43. [Google Scholar] [CrossRef]
- Ramlakhan, M.; Wu, C.Y.; Watano, S.; Dave, R.N.; Pfeffer, R. Dry particle coating using magnetically assisted impaction coating: Modification of surface properties and optimization of system and operating parameters. Powder Technol. 2000, 112, 137–148. [Google Scholar] [CrossRef]
- Pfeffer, R.; Dave, R.N.; Wei, D.; Ramlakhan, M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001, 117, 40–67. [Google Scholar]
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21–33. [Google Scholar] [CrossRef]
- Mullarney, M.P.; Beach, L.E.; Davé, R.N.; Langdon, B.A.; Polizzi, M.; Blackwood, D.O. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011, 212, 397–402. [Google Scholar] [CrossRef]
- Hentzschel, C.M.; Alnaief, M.; Smirnova, I.; Sakmann, A.; Leopold, C.S. Tableting properties of silica aerogel and other silicates. Drug Dev. Ind. Pharm. 2012, 38, 462–467. [Google Scholar] [CrossRef]
- Chattoraj, S.; Shi, L.; Sun, C.C. Profoundly improving flow properties of a cohesive cellulose powder by surface coating with nano-silica through comilling. J. Pharm. Sci. 2011, 100, 4943–4952. [Google Scholar] [CrossRef]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Davé, R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Suttiruengwong, S.; Seiler, M.; Arlt, W. Dissolution rate enhancement by adsorption of poorly soluble drugs on hydrophilic silica aerogels. Pharm. Dev. Technol. 2005, 9, 443–452. [Google Scholar] [CrossRef]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J. Non-Cryst. Solids 2004, 350, 54–60. [Google Scholar] [CrossRef]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.Y.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J. Drug delivery formulations of ordered and nonordered mesoporous silica: Comparison of three drug loading methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef] [PubMed]
- Bolko Seljak, K.; Ilić, I.G.; Gašperlin, M.; Zvonar Pobirk, A. Self-microemulsifying tablets prepared by direct compression for improved resveratrol delivery. Int. J. Pharm. 2018, 548, 263–275. [Google Scholar] [CrossRef]
- Mura, P.; Valleri, M.; Cirri, M.; Mennini, N. New solid self-microemulsifying systems to enhance dissolution rate of poorly water soluble drugs. Pharm. Dev. Technol. 2012, 17, 277–284. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Pawlak, S.A.; Dalrymple, D.M.; Nider, C.J.; Trombetta, L.D.; Serajuddin, A.T.M. Development of solid sedds, iv: Effect of adsorbed lipid and surfactant on tableting properties and surface structures of different silicates. Pharm. Res. 2013, 30, 3170–3185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiong, N.; Elkordy, A.A. Effects of liquisolid formulations on dissolution of naproxen. Eur. J. Pharm. Biopharm. 2009, 73, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Suitability of various excipients as carrier and coating materials for liquisolid compacts. Drug Dev. Ind. Pharm. 2011, 37, 1200–1207. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Z.; Quan, G.; Peng, X.; Pan, X.; Wang, R.; Xu, Y.; Li, G.; Wu, C. In vitro and in vivo evaluation of ordered mesoporous silica as a novel adsorbent in liquisolid formulation. Int. J. Nanomed. 2012, 7, 199. [Google Scholar]
- El-Houssieny, B.M.; Wahman, L.; Arafa, N.M. Bioavailability and biological activity of liquisolid compact formula of repaglinide and its effect on glucose tolerance in rabbits. Biosci. Trends 2010, 4, 17–24. [Google Scholar]
- Basalious, E.B.; El-Sebaie, W.; El-Gazayerly, O. Rapidly absorbed orodispersible tablet containing molecularly dispersed felodipine for management of hypertensive crisis: Development, optimization and in vitro/in vivo studies. Pharm. Dev. Technol. 2013, 18, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Fuji Chemical Industry Co., Ltd. Available online: http://www.fujichemical.co.jp/english/medical/medicine/neusilin/neusilin_brochure.pdf (accessed on 25 September 2020).
- Shete, A.; Salunkhe, A.; Yadav, A.; Sakhare, S.; Doijad, R. Neusilin based liquisolid compacts of albendazole: Design, development, characterization and in vitro anthelmintic activity. J. Res. Pharm. 2019, 23, 441–456. [Google Scholar] [CrossRef] [Green Version]
- Vranikova, B.; Gajdziok, J. Evaluation of sorptive properties of various carriers and coating materials for liquisolid systems. Acta Pol. Pharm. 2015, 72, 539–549. [Google Scholar]
- Dias, R.J.; Mali, K.K.; Ghorpade, V.S.; Havaldar, V.D.; Mohite, V.R. Formulation and evaluation of carbamazepine liquisolid compacts using novel carriers. Indian J. Pharm. Educ. Res. 2017, 51, S69–s78. [Google Scholar] [CrossRef] [Green Version]
- Krupa, A.; Szlęk, J.; Jany, B.R.; Jachowicz, R. Preformulation studies on solid self-emulsifying systems in powder form containing magnesium aluminometasilicate as porous carrier. AAPS PharmSciTech 2015, 16, 623–635. [Google Scholar] [PubMed] [Green Version]
- Cirri, M.; Mura, P.; Valleri, M.; Brunetti, L. Development and characterization of liquisolid tablets based on mesoporous clays or silicas for improving glyburide dissolution. Pharmaceutics 2020, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Omar, T.A.; Oka, S.; Muzzio, F.J.; Glasser, B.J. Manufacturing of pharmaceuticals by impregnation of an active pharmaceutical ingredient onto a mesoporous carrier: Impact of solvent and loading. J. Pharm. Innov. 2019, 14, 194–205. [Google Scholar] [CrossRef]
- Juneja, P.; Kaur, B.; Odeku, O.A.; Singh, I. Development of corn starch-neusilin ufl2 conjugate as tablet superdisintegrant: Formulation and evaluation of fast disintegrating tablets. J. Drug Deliv. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Helmis, M.; Mohamad, B.; Kumpugdee-Vollrath, M. Influence of several excipients on drug release of tablets containing resveratrol. Mathews J. Pharm. Sci. 2016, 1, 7. [Google Scholar]
- Fuji Chemical Industry Co., Ltd. Available online: http://www.fujichemical.co.jp/english/medical/medicine/neusilin/index.html (accessed on 25 September 2020).
- Maclean, J.; Medina, C.; Daurio, D.; Alvarez-Nunez, F.; Jona, J.; Munson, E.; Nagapudi, K. Manufacture and performance evaluation of a stable amorphous complex of an acidic drug molecule and neusilin. J. Pharm. Sci. 2011, 100, 3332–3344. [Google Scholar] [CrossRef]
- Kamel, R.; Basha, M. Preparation and in vitro evaluation of rutin nanostructured liquisolid delivery system. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Tseng, Y.-C.; Goldman, D.; Bogner, R.H. Hydrogen bonding with adsorbent during storage governs drug dissolution from solid-dispersion granules. Pharm. Res. 2002, 19, 1663–1672. [Google Scholar] [CrossRef]
- Gupta, M.K.; Goldman, D.; Bogner, R.H.; Tseng, Y.-C. Enhanced drug dissolution and bulk properties of solid dispersions granulated with a surface adsorbent. Pharm. Dev. Technol. 2001, 6, 563–572. [Google Scholar]
- Gupta, M.K.; Vanwert, A.; Bogner, R.H. Formation of physically stable amorphous drugs by milling with neusilin. J. Pharm. Sci. 2003, 92, 536–551. [Google Scholar] [CrossRef]
- Jones, T. The effect of glidant addition on the flowability of bulk particulate solids. J. Soc. Cosmet. Chem. 1970, 21, 483–500. [Google Scholar]
- Sindel, U.; Schweiger, A.; Zimmermann, I. Determination of the optimum mixing time for a mixture of lactose and colloidal silicon dioxide. J. Pharm. Sci. 1998, 87, 524–526. [Google Scholar] [PubMed]
- Lumay, G.; Pillitteri, S.; Marck, M.; Monsuur, F.; Pauly, T.; Ribeyre, Q.; Francqui, F.; Vandewalle, N. Influence of mesoporous silica on powder flow and electrostatic properties on short and long term. J. Drug Deliv. Sci. Technol. 2019, 53, 101192. [Google Scholar] [CrossRef]
- Sunkara, D.; Capece, M. Influence of material properties on the effectiveness of glidants used to improve the flowability of cohesive pharmaceutical powders. AAPS PharmSciTech 2018, 19, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Majerová, D.; Veselý, M.; Kulaviak, L.; Ruzicka, M.C.; Zámostný, P. On the mechanism of colloidal silica action to improve flow properties of pharmaceutical excipients. Int. J. Pharm. 2019, 556, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Yonemochi, E. Evaluation of the physical properties of dry surface-modified ibuprofen using a powder rheometer (ft4) and analysis of the influence of pharmaceutical additives on improvement of the powder flowability. Int. J. Pharm. 2020, 579, 119165. [Google Scholar] [CrossRef] [PubMed]
- Jonat, S.; Hasenzahl, S.; Gray, A.; Schmidt, P.C. Mechanism of glidants: Investigation of the effect of different colloidal silicon dioxide types on powder flow by atomic force and scanning electron microscopy. J. Pharm. Sci. 2004, 93, 2635–2644. [Google Scholar]
- Hurychová, H.; Kuentz, M.; Šklubalová, Z. Fractal aspects of static and dynamic flow properties of pharmaceutical excipients. J. Pharm. Innov. 2018, 13, 15–26. [Google Scholar] [CrossRef]
- Trpělková, Ž.; Hurychová, H.; Kuentz, M.; Vraníková, B.; Šklubalová, Z. Introduction of the energy to break an avalanche as a promising parameter for powder flowability prediction. Powder Technol. 2020, 375, 33–41. [Google Scholar] [CrossRef]
- Naiserova, M.; Kubova, K.; Vyslouzil, J.; Pavlokova, S.; Vetchy, D.; Urbanova, M.; Brus, J.; Vyslouzil, J.; Kulich, P. Investigation of dissolution behavior hpmc/eudragit®/magnesium aluminometasilicate oral matrices based on nmr solid-state spectroscopy and dynamic characteristics of gel layer. AAPS PharmSciTech 2018, 19, 681–692. [Google Scholar] [CrossRef]
- Tran, D.T.; Komínová, P.; Kulaviak, L.; Zámostný, P. Evaluation of multifunctional magnesium aluminosilicate materials as novel family of glidants in solid dosage products. Int. J. Pharm. 2020, 592, 120054. [Google Scholar] [CrossRef]
- Freeman, R.E.; Cooke, J.R.; Schneider, L.C.R. Measuring shear properties and normal stresses generated within a rotational shear cell for consolidated and non-consolidated powders. Powder Technol. 2009, 190, 65–69. [Google Scholar]
- Freeman, R.E. Measuring the flow properties of consolidated, conditioned and aerated powders—A comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007, 174, 25–33. [Google Scholar] [CrossRef]
- Schulze, D. Powders and Bulk Solids: Behavior, Characterization, Storage and Flow, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar]
- Dean, R.B.; Dixon, W.J. Simplified statistics for small numbers of observations. Anal. Chem. 1951, 23, 636–638. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Alderborn, G.; Pasanen, K.; Nyström, C. Studies on direct compression of tablets. Xl characterization of particle fragmentation during compaction by permeametry measurements of tablets. Int. J. Pharm. 1985, 23, 79–86. [Google Scholar] [CrossRef]
- Olsson, H.; Nyström, C. Assessing tablet bond types from structural features that affect tablet tensile strength. Pharm. Res. 2001, 18, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, Å.; Olsson, H.; Nyström, C. Effect of particle size and compaction load on interparticulate bonding structure for some pharmaceutical materials studied by compaction and strength characterisation in butanol. Eur. J. Pharm. Biopharm. 1997, 44, 243–251. [Google Scholar] [CrossRef]
- Adolfsson, Å.; Caramella, C.; Nyström, C. The effect of milling and addition of dry binder on the interparticulate bonding mechanisms in sodium chloride tablets. Int. J. Pharm. 1998, 160, 187–195. [Google Scholar] [CrossRef]
- Nyström, C.; Alderborn, G.; Duberg, M.; Karehill, P.-G. Bonding surface area and bonding mechanism-two important factors fir the understanding of powder comparability. Drug Dev. Ind. Pharm. 1993, 19, 2143–2196. [Google Scholar] [CrossRef]





| Notation | Material (w/w%) | ||||
|---|---|---|---|---|---|
| Microcrystalline Cellulose | MAS | ||||
| 100–150 µm | 150–250 µm | Neusilin® US2 | Neusilin® S2 | ||
| 1 | 99 | - | 1 | - | |
| 2 | 95 | - | 5 | - | |
| 3 | 90 | - | 10 | - | |
| 4 | 75 | - | 25 | - | |
| 5 | - | 99 | 1 | - | |
| 6 | - | 95 | 5 | - | |
| 7 | - | 90 | 10 | - | |
| 8 | - | 75 | 25 | - | |
| 9 | 99 | - | - | 1 | |
| 10 | 95 | - | - | 5 | |
| 11 | 90 | - | - | 10 | |
| 12 | - | 99 | - | 1 | |
| 13 | - | 95 | - | 5 | |
| 14 | - | 90 | - | 10 | |
| Material | Particle Size Distribution | Particle Shape | ||
|---|---|---|---|---|
| d10 (µm) | d50 (µm) | d90 (µm) | ||
| 100–150 µm MCC fraction | 99.7 | 162.0 | 242.0 | irregular |
| 150–250 µm MCC fraction | 132.0 | 229.0 | 341.0 | irregular |
| Neusilin® US2 | 46.1 | 108.0 | 206.6 | spherical |
| Neusilin® S2 | 64.2 | 140.1 | 249.3 | spherical |
| Applied Compaction Pressure (MPa) | 5 | 50 | 100 | 150 |
| Relative Standard Deviation RSD (%) | 5.35–23.97 | 1.25–12.06 | 2.32–9.67 | 1.68–10.45 |
| Glidant Concentration (w/w%) | Effective Angle of Internal Friction (°) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | 25 | ||
| 100–150 µm MCC fraction | Aerosil® 200 | 40.38 a | 32.85 a | 36.49 a | - | - |
| Neusilin® US2 | 33.28 | 31.33 | 30.05 | 24.55 | ||
| Neusilin® S2 | 34.46 | 35.63 | 31.53 | - | ||
| 150–250 µm MCC fraction | Aerosil® 200 | 31.66 a | 32.89 a | - | - | |
| Neusilin® US2 | 33.90 | 29.78 | 30.15 | 24.79 | ||
| Neusilin® S2 | 33.63 | 33.57 | 32.14 | - | ||
| Compound | Tensile Strength (MPa) - Applied Pressure 100 MPa | Tensile Strength (MPa) - Applied Pressure 150 MPa |
|---|---|---|
| 100–150 µm MCC fraction | 5.37 ± 0.30 | 7.07 ± 0.47 |
| 150–250 µm MCC fraction | 5.35 ± 0.11 | 7.05 ± 0.27 |
| Neusilin® US2 | 7.72 ± 0.46 | 9.62 ± 0.38 |
| Neusilin® S2 | 4.12 ± 0.40 | 6.37 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komínová, P.; Kulaviak, L.; Zámostný, P. Stress-Dependent Particle Interactions of Magnesium Aluminometasilicates as Their Performance Factor in Powder Flow and Compaction Applications. Materials 2021, 14, 900. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14040900
Komínová P, Kulaviak L, Zámostný P. Stress-Dependent Particle Interactions of Magnesium Aluminometasilicates as Their Performance Factor in Powder Flow and Compaction Applications. Materials. 2021; 14(4):900. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14040900
Chicago/Turabian StyleKomínová, Pavlína, Lukáš Kulaviak, and Petr Zámostný. 2021. "Stress-Dependent Particle Interactions of Magnesium Aluminometasilicates as Their Performance Factor in Powder Flow and Compaction Applications" Materials 14, no. 4: 900. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14040900