Facile Fabrication of ZnO-ZnFe2O4 Hollow Nanostructure by a One-Needle Syringe Electrospinning Method for a High-Selective H2S Gas Sensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparations
2.2. Preparations of ZnO-ZnFe2O4 Hollow Nanofibers
2.3. Material Characterizations
2.4. Gas Sensor Performance Measurement
3. Results
3.1. Structure and Surface Morphologies
3.2. TEM and XPS Analysis
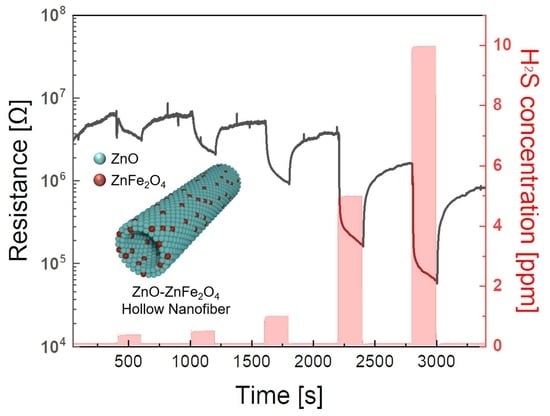
3.3. Gas Sensing Performances
3.4. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kyun Kim, H.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Khan, M.K.; Wu, F.; Chen, G.; Zeng, P. Millimeter-wave wireless communications for iot-cloud supported autonomous vehicles: Overview, design, and challenges. IEEE Commun. Mag. 2017, 55, 62–68. [Google Scholar] [CrossRef]
- Pirbhulal, S.; Zhang, H.; E Alahi, M.E.; Ghayvat, H.; Mukhopadhyay, S.C.; Zhang, Y.-T.; Wu, W. A novel secure iot-based smart home automation system using a wireless sensor network. Sensors 2017, 17, 69. [Google Scholar] [CrossRef]
- Hassan, K.; Tung, T.T.; Yap, P.L.; Rastin, H.; Stanley, N.; Nine, M.J.; Losic, D. Fractal design for advancing the performance of chemoresistive sensors. ACS Sens. 2021, 6, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Park, C.H.; Lee, J.; Lee, H.R.; Yang, C.; Oh, J.H. Chemically robust ambipolar organic transistor array directly patterned by photolithography. Adv. Mater. 2017, 29, 1605282. [Google Scholar] [CrossRef]
- Kang, H.; Cho, S.-Y.; Ryu, J.; Choi, J.; Ahn, H.; Joo, H.; Jung, H.-T. Multiarray nanopattern electronic nose (e-nose) by high-resolution top-down nanolithography. Adv. Funct. Mater. 2020, 30, 2002486. [Google Scholar] [CrossRef]
- Lewis, R.J.; Copley, G.B. Chronic low-level hydrogen sulfide exposure and potential effects on human health: A review of the epidemiological evidence. Crit. Rev. Toxicol. 2015, 45, 93–123. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.-H.; Kim, J.-H.; Mirzaei, A.; Woo Kim, H.; Kim, S.S. Realization of H2S sensing by Pd-functionalized networked CuO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Li, H.; Sun, M.; Han, S.; Cai, C.; Shen, W.; Fu, Y. Ultrafast response/recovery and high selectivity of the H2S gas sensor based on α-Fe2O3 nano-ellipsoids from one-step hydrothermal synthesis. ACS Appl. Mater. Interfaces 2019, 11, 12761–12769. [Google Scholar] [CrossRef]
- Al Shboul, A.M.; Izquierdo, R. Printed chemiresistive In2O3 nanoparticle-based sensors with ppb detection of H2S gas for food packaging. ACS Appl. Nano Mater. 2021, 4, 9508–9517. [Google Scholar] [CrossRef]
- Park, K.-R.; Cho, H.-B.; Lee, J.; Song, Y.; Kim, W.-B.; Choa, Y.-H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Choi, S.J.; Choi, C.; Kim, S.-J.; Cho, H.-J.; Hakim, M.; Jeon, S.; Kim, I.D. Highly efficient electronic sensitization of non-oxidized graphene flakes on controlled pore-loaded WO3 nanofibers for selective detection of H2S molecules. Sci. Rep. 2015, 5, 8067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, H.-B.; Zhang, X.-F.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Large-scale synthesis of hierarchically porous ZnO hollow tubule for fast response to ppb-level H2S gas. ACS Appl. Mater. Interfaces 2019, 11, 11627–11635. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Zhang, C. Zinc ferrite based gas sensors: A review. Ceram. Int. 2019, 45, 11143–11157. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Wang, C.; Sun, P.; Hu, X.; Li, X.; Shimanoe, K.; Yamazoe, N.; Lu, G. Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens. Actuators B Chem. 2015, 206, 577–583. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Yang, J.; Zhu, Z.; Zhang, H.; Wang, Y. Synthesis and gas sensor application of ZnFe2O4–ZnO composite hollow microspheres. RSC Adv. 2014, 4, 57967–57974. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, T.; Zhou, T.; Lou, Z.; Deng, J.; Wang, L. Fast and real-time acetone gas sensor using hybrid ZnFe2O4/ZnO hollow spheres. RSC Adv. 2016, 6, 66738–66744. [Google Scholar] [CrossRef]
- Abideen, Z.-U.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun Metal Oxide Composite Nanofibers Gas Sensors: A Review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Rianjanu, A.; Fauzi, F.; Triyana, K.; Wasisto, H.S. Electrospun Nanofibers for Quartz Crystal Microbalance Gas Sensors: A Review. ACS Appl. Nano Mater. 2021, 4, 9957–9975. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Peng, Y.; Zhao, C.; He, Y.; Zhang, Z.; Pan, X.; Mellors, N.J.; Xie, E. Wire-in-tube structure fabricated by single capillary electrospinning via nanoscale kirkendall effect: The case of nickel-zinc ferrite. Nanoscale 2013, 5, 12551–12557. [Google Scholar] [CrossRef]
- Ji, D.; Fan, L.; Tao, L.; Sun, Y.; Li, M.; Yang, G.; Tran, T.Q.; Ramakrishna, S.; Guo, S. The kirkendall effect for engineering oxygen vacancy of hollow Co3O4 nanoparticles toward high-performance portable zinc-air batteries. Angew. Chem. Int. Ed. 2019, 58, 13840–13844. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, M.; Yu, R. Surfactant-free synthesis of octahedral ZnO/ ZnFe2O4 heterostructure with ultrahigh and selective adsorption capacity of malachite green. Sci. Rep. 2016, 6, 25074. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Shao, R.; Tang, L.; Chen, Z. Synthesis of ZnFe2O4/ZnO nanocomposites immobilized on graphene with enhanced photocatalytic activity under solar light irradiation. J. Alloy. Compd. 2013, 564, 55–62. [Google Scholar] [CrossRef]
- Dhal, J.P.; Mishra, B.G.; Hota, G. Hydrothermal synthesis and enhanced photocatalytic activity of ternary Fe2O3/ ZnFe2O4/ZnO nanocomposite through cascade electron transfer. RSC Adv. 2015, 5, 58072–58083. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierarchical mesoporous Bi-component-active ZnO/ZnFe2O4 sub-microcubes as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.-H.; Chang, D.; Kim, S. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B Chem. 2010, 149, 28–33. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Hao, F.; Kang, C.; Cui, B.; Wei, D.; Meng, F. P-n junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. Appl. Surf. Sci. 2021, 538, 148140. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Yang., J.; Gao, X.; Zhang, H.; Wang, Y.; Zhu, Z. Spinel ZnFe2O4 nanoparticle-decorated rod-like ZnO nanoheterostructures for enhanced gas sensing performances. RSC Adv. 2015, 5, 10048–10057. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-R.; Kim, R.N.; Song, Y.; Kwon, J.; Choi, H. Facile Fabrication of ZnO-ZnFe2O4 Hollow Nanostructure by a One-Needle Syringe Electrospinning Method for a High-Selective H2S Gas Sensor. Materials 2022, 15, 399. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15020399
Park K-R, Kim RN, Song Y, Kwon J, Choi H. Facile Fabrication of ZnO-ZnFe2O4 Hollow Nanostructure by a One-Needle Syringe Electrospinning Method for a High-Selective H2S Gas Sensor. Materials. 2022; 15(2):399. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15020399
Chicago/Turabian StylePark, Kee-Ryung, Ryun Na Kim, Yoseb Song, Jinhyeong Kwon, and Hyeunseok Choi. 2022. "Facile Fabrication of ZnO-ZnFe2O4 Hollow Nanostructure by a One-Needle Syringe Electrospinning Method for a High-Selective H2S Gas Sensor" Materials 15, no. 2: 399. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15020399