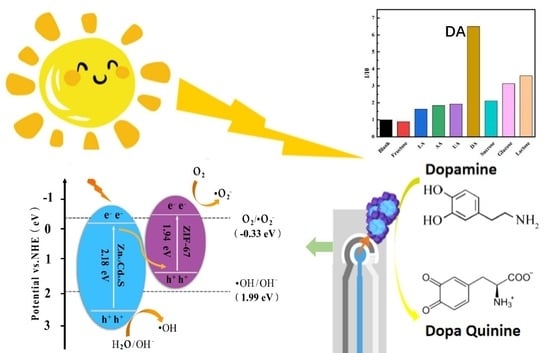
Heterostructured ZnCdS@ZIF-67 as a Photocatalyst for Fluorescent Dye Degradation and Selectively Nonenzymatic Sensing of Dopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-67
2.3. Synthesis of Zn0.2Cd0.8S@ZIF-67
2.4. Material Characterization
2.5. Photocatalytic Degradation Experiment
2.6. Free Radical Trapping Experiment
2.7. Fabrication of the DA Sensor
3. Results and Discussion
3.1. Morphological and Structural Characterizations of Zn0.2Cd0.8S@ZIF-67
3.2. Photoelectrochemical Performances and Analysis
3.3. Photocatalyst Degradation of RhB
3.4. DA Sensor and Mechanism Exploration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, J.S. Ruthenium Nanoparticle-Immobilized Porous Carbon Nanofibers for Nonenzymatic Dopamine Sensing. ACS Appl. Nano Mater. 2021, 4, 13683–13691. [Google Scholar] [CrossRef]
- Harley, C.C.; Rooney, A.D.; Breslin, C.B. The selective detection of dopamine at a polypyrrole film doped with sulfonated β-cyclodextrins. Sens. Actuators B Chem. 2010, 150, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Dashtian, K.; Hajati, S.; Ghaedi, M. Ti-Based Solid-State Imprinted-Cu2O/CuInSe2 Heterojunction Photoelectrochemical platform for Highly Selective Dopamine Monitoring. Sens. Actuators B Chem. 2021, 326, 128824. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Zhai, B.; Liang, Y. Enhanced photocatalytic degradation of RhB by two-dimensional composite photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 429–435. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Yang, X.; Peng, Y.-L.; Cheng, P.; Zhang, Z.; Chen, Y. Template-Directed Fabrication of Highly Efficient Metal-Organic Framework Photocatalysts. ACS Appl. Mater. Interfaces 2021, 13, 58619–58629. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, R.; Lou, J.; Yuan, J.; Xu, J.; Fan, X. Novel marigold-like CuO@Cu-based MOFs composite photocatalyst for high-performance removal of alkylphenol ethoxylate under visible light. J. Environ. Chem. Eng. 2021, 9, 106434. [Google Scholar] [CrossRef]
- Zou, X.-N.; Zhang, D.; Luan, T.-X.; Li, Q.; Li, L.; Li, P.-Z.; Zhao, Y. Incorporating Photochromic Triphenylamine into a Zirconium-Organic Framework for Highly Effective Photocatalytic Aerobic Oxidation of Sulfides. ACS Appl. Mater. Interfaces 2021, 13, 20137–20144. [Google Scholar] [CrossRef]
- La Ngoc Tran, N.; Phan, B.T.; Ta, H.K.T.; Chi, T.T.K.; Hien, B.T.T.; Phuong, N.T.T.; Nguyen, C.C.; Doan, T.L.H.; Tran, N.H.T. Gold nanoparticles are capped under the IRMOF-3 platform for in-situ surface-enhanced Raman scattering technique and optic fiber sensor. Sens. Actuators A Phys. 2022, 347, 113932. [Google Scholar] [CrossRef]
- He, H.; Li, R.; Yang, Z.; Chai, L.; Jin, L.; Alhassan, S.I.; Ren, L.; Wang, H.; Huang, L. Preparation of MOFs and MOFs derived materials and their catalytic application in air pollution: A review. Catal. Today 2021, 375, 10–29. [Google Scholar] [CrossRef]
- Li-Li, Z.; Liu, M.; Ke-An, W.; Hai-Bin, Z. Assembling Ag/UiO-66-NH2 Composites for Photocatalytic Dye Degradation. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1896–1901. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Jiang, Y.; Liao, J.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Construction of a ternary WO3/CsPbBr3/ZIF-67 heterostructure for enhanced photocatalytic carbon dioxide reduction. Sci. China Mater. 2022, 65, 1550–1559. [Google Scholar] [CrossRef]
- Kumar, O.P.; Ahmad, M.; Nazir, M.A.; Anum, A.; Jamshaid, M.; Shah, S.S.A.; Rehman, A. Strategic combination of metal-organic frameworks and C3N4 for expeditious photocatalytic degradation of dye pollutants. Environ. Sci. Pollut. Res. 2022, 29, 35300–35313. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Imran, M.; Aqeel, M.; Naz, M.; Ikram, M.; Ali, S. Study of Transition Metal Ion Doped CdS Nanoparticles for Removal of Dye from Textile Wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1915–1923. [Google Scholar] [CrossRef]
- Junaid, M.; Imran, M.; Ikram, M.; Naz, M.; Aqeel, M.; Afzal, H.; Majeed, H.; Ali, S. The study of Fe-doped CdS nanoparticle-assisted photocatalytic degradation of organic dye in wastewater. Appl. Nanosci. 2019, 9, 1593–1602. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, J.; Song, Y.; Chen, Y.; Lu, F.; Gao, W. Porous hollow carbon nanobubbles@ZnCdS multi-shelled dodecahedral cages with enhanced visible-light harvesting for ultrasensitive photoelectrochemical biosensors. Biosens. Bioelectron. 2019, 133, 125–132. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, Y.; Hao, X. Self-assembly of zinc cadmium sulfide nanorods into nanoflowers with enhanced photocatalytic hydrogen production activity. J. Colloid Interface Sci. 2020, 567, 357–368. [Google Scholar] [CrossRef]
- Liu, K.; Peng, L.; Zhen, P.; Chen, L.; Song, S.; Garcia, H.; Sun, C. ZnCdS Dotted with Highly Dispersed Pt Supported on SiO2 Nanospheres Promoting Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2021, 125, 14656–14665. [Google Scholar] [CrossRef]
- Chen, M.; Huang, X.; Chen, C.; Hou, W.; Xu, Y. M-Dependent activity of MCo2O4 spinels for water splitting and H2 production on Zn0.5Cd0.5S under visible light. Appl. Catal. B-Environ. 2021, 298, 120469. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, J.; Ding, Y.; Zheng, D.; Lin, Y.; Chen, Y.; Gao, W. Rationally designed hierarchical hollow ZnCdS@MoS2 heterostructured cages with efficient separation of photogenerated carriers for photoelectrochemical aptasensing of lincomycin. Sens. Actuators B-Chem. 2020, 306, 127552. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.-A.; Chen, R.; Wu, C.-Y.; Yu, Y.-Q.; Xu, J.; Hu, J.-G.; Luo, L.-B. Gallium doped n-type ZnxCd1−xS nanoribbons: Synthesis and photoconductivity properties. J. Appl. Phys. 2014, 115, 063108. [Google Scholar] [CrossRef]
- Yan, X.; Jin, Z.; Zhang, Y.; Zhang, Y.; Yuan, H. Sustainable and efficient hydrogen evolution over a noble metal-free WP double modified ZnxCd1−xS photocatalyst driven by visible-light. Dalton Trans. 2019, 48, 11122–11135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, C.; Ma, H.; Li, G.; Chen, L.; Sun, Y.; Chen, Z.; Fang, P.; Fu, Q.; Pan, C. Synthesis of flower-liked twin crystal ternary Ni/NiS/Zn0.2Cd0.8S catalyst for highly efficient hydrogen production. Chem. Eng. J. 2021, 406, 126878. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Xu, L.; Jin, Z.; Wang, Y. Facile synthesis of difunctional NiV LDH@ZIF-67 p-n junction: Serve as prominent photocatalyst for hydrogen evolution and supercapacitor electrode as well. Renew. Energy 2020, 162, 535–549. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, S.; Xu, S.; She, Y.; Li, Y.; Leveneur, S.; Qin, Y. Piezoelectric effect synergistically enhances the performance of Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2021, 291, 120019. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, F.; Feng, L.; Huang, J.; Kong, X.; Zhang, H.; Li, H.; Wang, X. Constructing 3D hierarchical Zn0.2Cd0.8S microspheres for the improved visible-light-driven photocatalytic performance. Int. J. Hydrogen Energy 2019, 44, 23868–23879. [Google Scholar] [CrossRef]
- Guo, H.; Ding, J.; Wan, S.; Wang, Y.; Zhong, Q. Highly efficient CH3OH production over Zn0.2Cd0.8S decorated g-C3N4 heterostructures for the photoreduction of CO2. Appl. Surf. Sci. 2020, 528, 146943. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Li, Y.; Duan, J.; Zhang, G.; Hu, L.; Wang, X.; Zhang, W. Visible-light-driven photo-Fenton reactions using Zn1−1.5xFexS/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis. Appl. Catal. B-Environ. 2020, 266, 118653. [Google Scholar] [CrossRef]
- Yu, H.; Xu, S.; Zhang, S.; Wang, S.; He, Z. In-situ construction of core–shell structured TiB2-TiO2@g-C3N4 for efficient photocatalytic degradation. Appl. Surf. Sci. 2022, 579, 152201. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, D.; Zhou, H.; Pi, M.; Wang, X.; Chen, S. Coupling P Nanostructures with P-Doped g-C3N4 As Efficient Visible Light Photocatalysts for H2 Evolution and RhB Degradation. ACS Sustain. Chem. Eng. 2018, 6, 6342–6349. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, N.; Luo, M.; Fan, L.; Zhao, K.; Qu, J.; Guan, J.; Yuan, X. Enhancement mechanism of fiddlehead-shaped TiO2-BiVO4 type II heterojunction in SPEC towards RhB degradation and detoxification. Appl. Surf. Sci. 2019, 463, 234–243. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, H.; Bai, K.; Chen, X.; Li, E.; Shen, Y.; Wang, M. Fabrication of a g-C3N4/MoS2 photocatalyst for enhanced RhB degradation. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 144, 115361. [Google Scholar] [CrossRef]
- Frindy, S.; Sillanpää, M. Synthesis and application of novel α-Fe2O3/graphene for visible-light enhanced photocatalytic degradation of RhB. Mater. Des. 2020, 188, 108461. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wen, B.; Zhou, J.; Zhao, X.; Zhang, X.; Su, Z. Heterostructured ZnCdS@ZIF-67 as a Photocatalyst for Fluorescent Dye Degradation and Selectively Nonenzymatic Sensing of Dopamine. Materials 2022, 15, 7683. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15217683
Wang Z, Wen B, Zhou J, Zhao X, Zhang X, Su Z. Heterostructured ZnCdS@ZIF-67 as a Photocatalyst for Fluorescent Dye Degradation and Selectively Nonenzymatic Sensing of Dopamine. Materials. 2022; 15(21):7683. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15217683
Chicago/Turabian StyleWang, Zhichao, Bianying Wen, Jie Zhou, Xin Zhao, Xiaoyuan Zhang, and Zhiqiang Su. 2022. "Heterostructured ZnCdS@ZIF-67 as a Photocatalyst for Fluorescent Dye Degradation and Selectively Nonenzymatic Sensing of Dopamine" Materials 15, no. 21: 7683. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15217683