Modulated Laser Cladding of Implant-Type Coatings by Bovine-Bone-Derived Hydroxyapatite Powder Injection on Ti6Al4V Substrates—Part I: Fabrication and Physico-Chemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
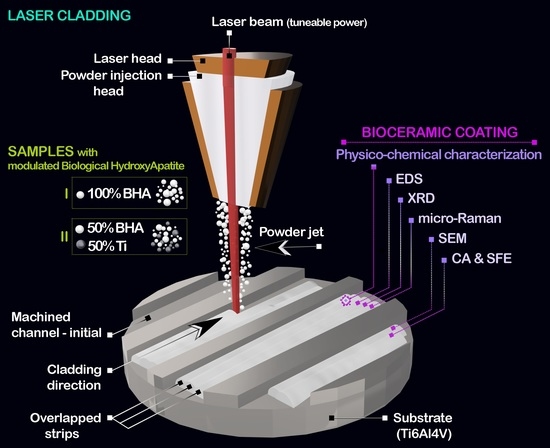
2.1. Sample Preparation
2.2. Sample Characterization
- (1)
- The chemical composition was determined by energy dispersive X-ray spectroscopy (EDS) using a microanalysis system (EDAX Sapphire UTW, 128 eV resolution, AMETEK Inc., Berwyn, PA, USA) attached to the scanning electron microscope, in five randomly selected areas of each specimen.
- (2)
- The crystalline status of the laser-cladded samples was investigated by X-ray diffraction (XRD) in symmetric (θ–θ) geometry using a Rigaku SmartLab 3 kW system (Rigaku Corporation, Tokyo, Japan) with CuKα radiation (λ = 1.5418 Å). The diffractometer was equipped with an HyPix-3000 detector, operated in 1D mode. The XRD measurements were conducted in parallel beam setting, in the 2θ range 20–90° with a step size of 0.02° and speed of 3 degrees/min.
- (3)
- In situ micro-Raman experiments were carried out using a LabRAM HR Evolution confocal spectrometer (Horiba Jobin-Yvon, Edison, NJ, USA) to analyze the structural properties of the samples based on their vibrational signatures. A He-Cd laser with a wavelength of 325 nm was focalized in backscattering geometry on the surface of the samples with a Thorlabs LMU-40× objective (Thorlabs Inc., Newton, NJ, USA). The Raman spectra were calibrated using the Rayleigh (0 cm–1) and silicon (520.7 cm–1) standard bands. The scattered light was recorded at different laser powers in the 150–2000 cm–1 spectral range using 2400 lines/mm diffraction grating. The spectral resolution was ~1 cm–1.
- (4)
- The morphological characteristics were evaluated by scanning electron microscopy (SEM), using a Phillips XL 30 ESEM TMP microscope (FEI/Phillips, Hillsboro, OR, USA). The acquisition of the micrographs was conducted at an acceleration voltage of 25 kV and a working distance of 10 mm, in five randomly chosen areas.
- (5)
- The wettability features were evaluated by water contact angle measurements using a Krüss Drop Shape Analyzer—DSA100 (A. Krüss Optronic GmbH, Hamburg, Germany). The experiments involved three wetting agents (water and ethylene glycol (EG) as polar agents, and diiodomethane (DIM) as a dispersive agent) and controlled ambient parameters (temperature of 20 ± 1 °C and room humidity of 45 ± 5%). The images were captured 1 s after the deposition of the wetting agent droplet. The results were processed with the ImageJ 1.50 software (National Institutes of Health, Bethesda, MD, USA) and an average of 5 determinations/sample were performed. The surface free energy was computed by the Owens, Wendt, Rabel, and Kaelble (OWRK) method [48].
3. Results and Discussion
3.1. Compositional Evaluation
3.2. XRD Investigations
3.3. Raman Spectroscopy Analysis
- ▪
- ▪
- ▪
- The Ti–O symmetric stretching mode, formed as a single peak for samples obtained at 500 W beam power with 100 wt.% BHA (664 cm–1) and 50 wt.% BHA (668 cm–1), and as a split-peak under increased laser power, at 638–650 cm–1 and 640–661 cm–1 for non-diluted and diluted BHA, respectively [55,56,57,59,60]. The second order contribution for this peak was attributed to the isolated CaO Raman mode (680–685 cm–1) as a result of BHA decomposition, in agreement with some previous reports [61] and the XRD data presented within;
- ▪
- The Ti–O out-of-plane mode, evidenced as maximum frequency bands at 802–808 cm–1 (100 wt.% BHA) and 801–803 cm–1 (50 wt.% BHA) [60,62]. As a laser-power-dependent advent in the 500–600 W range, the progressively elevated intensities of the peaks exposed the CaTiO3 phase tendency to become more stable when high temperatures are reached [49]. However, at a higher beam power, the development was inversely switched, endorsing the XRD findings in this respect.
- ▪
- the ν2 and ν4 bending modes of the O–P–O functional group identified at 382–389 cm−1 and 539–556 cm−1, and 381–389 cm−1 and 541 cm−1, for the 100 wt.% BHA and 50 wt.% BHA samples, respectively;
- ▪
- the ν1 bending mode and ν3 stretching mode of the orthophosphate functional groups, encountered at 1042–1051 cm−1 and 1095–1103 cm−1, and 1016 cm−1, 1043–1051 cm−1 and 1076 cm−1, for the 100 wt.% BHA and 50 wt.% BHA samples, respectively. However, the emergence of the typical vibrational bands of the ν3 stretching (965–1100 cm−1) mode of the (PO4)3− group, arising from the BHA phase, are overlapped on the same spectral region [13,63,64].
3.4. Morphological Evaluation
3.5. Contact Angle Measurements
4. Conclusions
- The low beam power regime allows for the conservation of the BHA phase and the formation of the biocompatible CaTiO3 compound.
- The high beam power regimes entail high temperatures, and lead to the partial decomposition of BHA and the advent of the biocompatible and soluble TTCP phase.
- Regardless of the laser beam power, yet with a slight preference towards the samples prepared using the 50/50 wt.% BHA/Ti ratio, the appearance of Ti5P3.31 was also noticed, along with a series of Ti oxides and sub-oxides and CaO phases, at the concentrational expense of the ubiquitous CaTiO3 phase.
- The surface of the cladded layers was marked by the formation of circular marks/grooves and overlapping strips in the direction in which the laser beam passed, independent of the BHA/Ti ratio or the laser beam power. The detailed examination exposed varied surface textures due to the formation of aggregates that gradually increased in number and size above 600 W for the 100 wt.% BHA samples and independently of the laser power for the 50 wt.% BHA samples.
- As a direct consequence of the excessive beam power, some BHA particles were incompletely homogenized.
- Favorably, the phase composition and morphological features induced the overall hydrophilic behavior of all samples, which is required for future biomedical applications.
- The laser cladding by powder injection process was demonstrated to be highly effective in creating a strong metallurgical bond between the metallic substrate and the ceramic coating.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chien, C.-S.; Liao, T.-Y.; Hong, T.-F.; Kuo, T.-Y.; Chang, C.-H.; Yeh, M.-L.; Lee, T.-M.; Cheng, Y. Surface microstructure and bioactivity of hydroxyapatite and fluorapatite coatings deposited on Ti-6Al-4V substrates using Nd-YAG laser. J. Med. Biol. Eng. 2014, 34, 109–115. [Google Scholar] [CrossRef]
- Manjaiah, M.; Laubscher, R.F. A review of the surface modifications of titanium alloys for biomedical applications. Mater. Tehnol. 2017, 51, 181–193. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, M.; Machedon-Pisu, T.; Maidaniuc, A.; Ciocoiu, R.C.; Ioniță, M.; Pasuk, I.; Stan, G.E.; Miculescu, F. Internal and external surface features of newly developed porous ceramics with random interconnected 3D channels by a fibrous sacrificial porogen method. Appl. Surf. Sci. 2019, 489, 226–238. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, T.; Evis, Z.; Tezcaner, A.; Aydınol, M.K. Effects of surface pretreatments and coating period on hydroxyapatite coating of Ti6Al4V alloy. J. Aust. Ceram. Soc. 2020, 56, 545–557. [Google Scholar] [CrossRef]
- Pradhan, D.; Wren, A.; Misture, S.; Mellott, N. Investigating the structure and biocompatibility of niobium and titanium oxides as coatings for orthopedic metallic implants. Mater. Sci. Eng. C 2016, 58, 918–926. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Bajda, S.; Liu, Y.; Triantaphyllou, A.; Rainforth, W.M.; Glendenning, M. 3D analysis of thermal and stress evolution during laser cladding of bioactive glass coatings. J. Mech. Behav. Biomed. Mater. 2016, 59, 404–417. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, F.; Stan, G.E.; Ciocoiu, R.-C.; Corobea, M.C.; Miculescu, M.; Ciocan, L.T. Preliminary Studies on Graphene-Reinforced 3D Products Obtained by the One-Stage Sacrificial Template Method for Bone Reconstruction Applications. J. Funct. Biomater. 2021, 12, 13. [Google Scholar] [CrossRef]
- Mitran, V.; Ion, R.; Miculescu, F.; Necula, M.; Mocanu, A.-C.; Stan, G.; Antoniac, I.; Cimpean, A. Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials. Materials 2018, 11, 1097. [Google Scholar] [CrossRef]
- Mindroiu, M.; Cicek, E.; Miculescu, F.; Demetrescu, I. The influence of thermal oxidation treatment on the electrochemical stability of TiAlV and TiAlFe alloys and their potential application as biomaterials. Rev. Chim. 2007, 58, 898–903. [Google Scholar]
- Kumar, S.; Mandal, A. Laser Cladding of Titanium Alloy. In Laser Cladding of Metals; Springer: Berlin/Heidelberg, Germany, 2021; pp. 215–242. [Google Scholar] [CrossRef]
- Weng, F.; Chen, C.; Yu, H. Research status of laser cladding on titanium and its alloys: A review. Mater. Des. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Nakhi, A.; Ganjali, M.; Shirinzadeh, H.; Sedaghat Ahangari Hossein Zadeh, A.; Mozafari, M. Laser Cladding of Fluorapatite Nanopowders on Ti6Al4V. Adv. Mater. Lett. 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros. Rev. 2016, 34, 65–83. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ko, C.-L.; Lin, D.-J.; Wu, H.-Y.; Hung, C.-C.; Chen, W.-C. In vivo studies of titanium implant surface treatment by sandblasted, acid-etched and further anchored with ceramic of tetracalcium phosphate on osseointegration. J. Aust. Ceram. Soc. 2019, 55, 799–806. [Google Scholar] [CrossRef]
- Behera, R.R.; Hasan, A.; Sankar, M.R.; Pandey, L.M. Laser cladding with HA and functionally graded TiO2-HA precursors on Ti–6Al–4V alloy for enhancing bioactivity and cyto-compatibility. Surf. Coat. Technol. 2018, 352, 420–436. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Surmeneva, M.; Vladescu, A.; Surmenev, R.; Pantilimon, C.; Braic, M.; Cotrut, C. Study on a hydrophobic Ti-doped hydroxyapatite coating for corrosion protection of a titanium based alloy. RSC Adv. 2016, 6, 87665–87674. [Google Scholar] [CrossRef]
- Rau, J.; Antoniac, I.; Filipescu, M.; Cotrut, C.; Fosca, M.; Nistor, L.; Birjega, R.; Dinescu, M. Hydroxyapatite coatings on Mg-Ca alloy prepared by pulsed laser deposition: Properties and corrosion resistance in simulated body fluid. Ceram. Int. 2018, 44, 16678–16687. [Google Scholar] [CrossRef]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptyug, A.; Kurtukova, M.O. Influence of the electrolyte’s pH on the properties of electrochemically deposited hydroxyapatite coating on additively manufactured Ti64 alloy. Sci. Rep. 2017, 7, 16819. [Google Scholar] [CrossRef]
- Lusquiños, F.; Pou, J.; Boutinguiza, M.; Quintero, F.; Soto, R.; León, B.; Pérez-Amor, M. Main characteristics of calcium phosphate coatings obtained by laser cladding. Appl. Surf. Sci. 2005, 247, 486–492. [Google Scholar] [CrossRef]
- Pascu, A.; Stanciu, E.; Roată, I.; Croitoru, C.; Balteş, L.; Tierean, M. Parameters and behaviour of NiCrFeSiB laser cladding in overlapped geometry. Bull. Transilv. Univ. Bras. Ser. I Eng. Sci. 2016, 9, 9–16. [Google Scholar]
- Pascu, A.; Stanciu, E.; Croitoru, C.; Roata, I.; Tierean, M. Pulsed Laser Cladding of Ni Based Powder. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012058. [Google Scholar] [CrossRef]
- Chien, C.-S.; Liu, C.-W.; Kuo, T.-Y. Effects of laser power level on microstructural properties and phase composition of laser-clad fluorapatite/zirconia composite coatings on Ti6Al4V substrates. Materials 2016, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Q. Effects of Diatomite Contents on Microstructure, Microhardness, Bioactivity and Biocompatibility of Gradient Bioceramic Coating Prepared by Laser Cladding. Metals 2022, 12, 931. [Google Scholar] [CrossRef]
- Avci, M.; Yilmaz, B.; Tezcaner, A.; Evis, Z. Strontium doped hydroxyapatite biomimetic coatings on Ti6Al4V plates. Ceram. Int. 2017, 43, 9431–9436. [Google Scholar] [CrossRef]
- Ganjali, M.; Mousavi, S.; Nikzamir, S.; Milan, P.B.; Mozafari, M. Effect of laser cladded co-doped strontium fluorapatite nanopowder coating on the antibacterial and cell attachment of Ti-6Al-4V implants for bone applications. Mater. Technol. 2022, 37, 829–841. [Google Scholar] [CrossRef]
- Liu, B.; Deng, Z.; Liu, D. Preparation and Properties of Multilayer Ca/P Bio-Ceramic Coating by Laser Cladding. Coatings 2021, 11, 891. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Bao, Z.; Dong, J.; Geng, Y.; Liu, S.; Wang, C.; Nie, P. Characterization of microstructure and mechanical properties of titanium-based bioactive ceramics laser-deposited on titanium alloy. Ceram. Int. 2022, 48, 28678–28691. [Google Scholar] [CrossRef]
- Büyüksağiş, A.; Çiftçi, N. HAP Coatings for Biomedical Applications: Biocompatibility and Surface Protection Against Corrosion of Ti, Ti6Al4V and AISI 316L SS. Prot. Met. Phys. Chem. Surf. 2020, 56, 834–843. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, F.; Miculescu, M.; Ciocoiu, R.C.; Pandele, A.M.; Stan, G.E.; Cîmpean, A.; Voicu, Ș.I.; Ciocan, L.-T. Comprehensive analysis of compatible natural fibre as sacrificial porogen template for tailored ceramic 3D bioproducts destined for hard tissue reconstruction. Ceram. Int. 2020, 47, 5318–5334. [Google Scholar] [CrossRef]
- DileepKumar, V.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Liu, S.-M.; Ko, C.-L.; Chen, W.-C. Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review. Polymers 2022, 14, 976. [Google Scholar] [CrossRef] [PubMed]
- Fouly, A.; Ibrahim, A.M.M.; Sherif, E.-S.M.; FathEl-Bab, A.M.; Badran, A. Effect of low hydroxyapatite loading fraction on the mechanical and tribological characteristics of poly (methyl methacrylate) nanocomposites for dentures. Polymers 2021, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.-C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.-C.; Voicu, Ș.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Miculescu, F.; Ciocan, L.; Miculescu, M.; Ernuteanu, A. Effect of heating process on micro structure level of cortical bone prepared for compositional analysis. Dig. J Nanomater. Biostruct. 2011, 6, 225–233. [Google Scholar]
- Miculescu, F.; Stan, G.; Ciocan, L.; Miculescu, M.; Berbecaru, A.; Antoniac, I. Cortical bone as resource for producing biomimetic materials for clinical use. Dig. J. Nanomater. Biostruct. 2012, 7, 1667–1677. [Google Scholar]
- Jing, Z.; Cao, Q.; Jun, H. Corrosion, wear and biocompatibility of hydroxyapatite bio-functionally graded coating on titanium alloy surface prepared by laser cladding. Ceram. Int. 2021, 47, 24641–24651. [Google Scholar] [CrossRef]
- Ganjali, M.; Ganjali, M.; Sadrnezhaad, S.; Pakzad, Y. Laser cladding of Ti alloys for biomedical applications. In Laser Cladding of Metals; Springer: Berlin/Heidelberg, Germany, 2021; pp. 265–292. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.; Ciocan, L.; Niculescu, M.; Corobea, M.; Rada, M.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Dan Batalu, N.; Cătălin Ciocoiu, R.; Voicu, S.t.I.; Stan, G.E.; Thakur, V.K. Synthesis and characterization of jellified composites from bovine bone-derived hydroxyapatite and starch as precursors for robocasting. ACS Omega 2018, 3, 1338–1349. [Google Scholar] [CrossRef]
- Miculescu, F.; Jepu, I.; Porosnicu, C.; Lungu, C.; Miculescu, M.; Burhala, B. A study on the influence of the primary electron beam on nanodimensional layers analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 335–345. [Google Scholar]
- Miculescu, F.; Bojin, D.; Ciocan, L.; Antoniac, I.; Miculescu, M.; Miculescu, N. Experimental researches on biomaterial-tissue interface interactions. J. Optoelectron. Adv. Mater. 2007, 9, 3303–3306. [Google Scholar]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Fredriksson, G.; Yadroitsev, I.; Krakhmalev, P. Fracture mechanisms in the as-built and stress-relieved laser powder bed fusion Ti6Al4V ELI alloy. Opt. Laser Technol. 2019, 109, 608–615. [Google Scholar] [CrossRef]
- Pascu, A.; Hulka, I.; Tierean, M.H.; Croitoru, C.; Stanciu, E.M.; Roată, I.C. A comparison of flame coating and laser cladding using Ni based powders. Solid State Phenom. 2016, 254, 77–82. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Yu, C.X.; Li, Y.; Zhu, Q.Q.; Zhou, L.; Cao, C.; Yu, T.T.; Du, F.P. Research on the changes in wettability of rice (Oryza sativa.) leaf surfaces at different development stages using the OWRK method. Pest Manag. Sci. 2014, 70, 462–469. [Google Scholar] [CrossRef]
- Chakraborty, R.; Raza, M.S.; Datta, S.; Saha, P. Synthesis and characterization of nickel free titanium–hydroxyapatite composite coating over Nitinol surface through in-situ laser cladding and alloying. Surf. Coat. Technol. 2019, 358, 539–550. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, F.; Stan, G.E.; Pandele, A.-M.; Pop, M.A.; Ciocoiu, R.C.; Voicu, Ș.I.; Ciocan, L.-T. Fiber-Templated 3D Calcium-Phosphate Scaffolds for Biomedical Applications: The Role of the Thermal Treatment Ambient on Physico-Chemical Properties. Materials 2021, 14, 2198. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Ciocoiu, R.C.; Butte, T.M.; Pasuk, I.; Stan, G.E.; Voicu, S.I.; Ciocan, L.T. Effect of the processing parameters on surface, physico-chemical and mechanical features of bioceramics synthesized from abundant carp fish bones. Ceram. Int. 2020, 46, 10159–10171. [Google Scholar] [CrossRef]
- Jillavenkatesa, A.; Condrate, R., Sr. The infrared and Raman spectra of tetracalcium phosphate (Ca4P2O9). Spectrosc. Lett. 1997, 30, 1561–1570. [Google Scholar] [CrossRef]
- Tlotleng, M.; Akinlabi, E.; Shukla, M.; Pityana, S. Microstructures, hardness and bioactivity of hydroxyapatite coatings deposited by direct laser melting process. Mater. Sci. Eng. C 2014, 43, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, U.t.; Eror, N. Laser-induced Raman scattering in calcium titanate. Solid State Commun. 1982, 44, 815–818. [Google Scholar] [CrossRef]
- Cavalcante, L.; Marques, V.; Sczancoski, J.; Escote, M.; Joya, M.; Varela, J.A.; Santos, M.; Pizani, P.; Longo, E. Synthesis, structural refinement and optical behavior of CaTiO3 powders: A comparative study of processing in different furnaces. Chem. Eng. J. 2008, 143, 299–307. [Google Scholar] [CrossRef]
- Oliveira, L.H.; Savioli, J.; de Moura, A.P.; Nogueira, I.C.; Li, M.S.; Longo, E.; Varela, J.A.; Rosa, I.L. Investigation of structural and optical properties of CaTiO3 powders doped with Mg2+ and Eu3+ ions. J. Alloys Compd. 2015, 647, 265–275. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Lo Vecchio, C.; Danyliv, O.; Baglio, V.; Martinelli, A.; Navarra, M.A. Composite Nafion-CaTiO3-δ membranes as electrolyte component for PEM fuel cells. Polymers 2020, 12, 2019. [Google Scholar] [CrossRef]
- Zheng, H.; de Györgyfalva, G.C.; Quimby, R.; Bagshaw, H.; Ubic, R.; Reaney, I.; Yarwood, J. Raman spectroscopy of B-site order–disorder in CaTiO3-based microwave ceramics. J. Eur. Ceram. Soc. 2003, 23, 2653–2659. [Google Scholar] [CrossRef]
- Zheng, H.; Reaney, I.; de Györgyfalva, G.C.; Ubic, R.; Yarwood, J.; Seabra, M.; Ferreira, V. Raman spectroscopy of CaTiO3-based perovskite solid solutions. J. Mater. Res. 2004, 19, 488–495. [Google Scholar] [CrossRef]
- Schmid, T.; Dariz, P. Shedding light onto the spectra of lime: Raman and luminescence bands of CaO, Ca(OH)2 and CaCO3. J. Raman Spectrosc. 2015, 46, 141–146. [Google Scholar] [CrossRef]
- Smolensky, G.; Siny, I.; Pisarev, R.; Kuzminov, E. Raman scattering in ordered and disordered perovskite type crystals. Ferroelectrics 1976, 12, 135–136. [Google Scholar] [CrossRef]
- Bramowicz, M.; Braic, L.; Azem, F.A.; Kulesza, S.; Birlik, I.; Vladescu, A. Mechanical properties and fractal analysis of the surface texture of sputtered hydroxyapatite coatings. Appl. Surf. Sci. 2016, 379, 338–346. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Stan, G.; Husanu, M.; Mercioniu, I.; Santos, L.; Fernandes, H.; Ferreira, J. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C. 2016, 62, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Schmid, M.; Hauser, C. Validation of a novel technique and evaluation of the surface free energy of food. Foods 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Terpiłowski, K.; Hołysz, L.; Chodkowski, M.; Clemente Guinarte, D. What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids Interfaces 2021, 5, 4. [Google Scholar] [CrossRef]
- Fernández, V.; Khayet, M. Evaluation of the surface free energy of plant surfaces: Toward standardizing the procedure. Front. Plant Sci. 2015, 6, 510. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, H.; Li, G.; Zhang, M.; Xiang, K.; Zhu, Z.; Wan, Y. Calcium titanate micro-sheets scaffold for improved cell viability and osteogenesis. Chem. Eng. J. 2020, 389, 124400. [Google Scholar] [CrossRef]
- Kim, S.H.; Ha, H.J.; Ko, Y.K.; Yoon, S.J.; Rhee, J.M.; Kim, M.S.; Lee, H.B.; Khang, G. Correlation of proliferation, morphology and biological responses of fibroblasts on LDPE with different surface wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar] [CrossRef]
- Stan, G.E.; Tite, T.; Popa, A.-C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D. The Beneficial Mechanicals and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings 2020, 10, 1119. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, A.-C.; Miculescu, F.; Stan, G.E.; Pasuk, I.; Tite, T.; Pascu, A.; Butte, T.M.; Ciocan, L.-T. Modulated Laser Cladding of Implant-Type Coatings by Bovine-Bone-Derived Hydroxyapatite Powder Injection on Ti6Al4V Substrates—Part I: Fabrication and Physico-Chemical Characterization. Materials 2022, 15, 7971. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15227971
Mocanu A-C, Miculescu F, Stan GE, Pasuk I, Tite T, Pascu A, Butte TM, Ciocan L-T. Modulated Laser Cladding of Implant-Type Coatings by Bovine-Bone-Derived Hydroxyapatite Powder Injection on Ti6Al4V Substrates—Part I: Fabrication and Physico-Chemical Characterization. Materials. 2022; 15(22):7971. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15227971
Chicago/Turabian StyleMocanu, Aura-Cătălina, Florin Miculescu, George E. Stan, Iuliana Pasuk, Teddy Tite, Alexandru Pascu, Tudor Mihai Butte, and Lucian-Toma Ciocan. 2022. "Modulated Laser Cladding of Implant-Type Coatings by Bovine-Bone-Derived Hydroxyapatite Powder Injection on Ti6Al4V Substrates—Part I: Fabrication and Physico-Chemical Characterization" Materials 15, no. 22: 7971. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15227971