Mechanical and Water Absorption Properties of Waterborne Polyurethane/Graphene Oxide Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
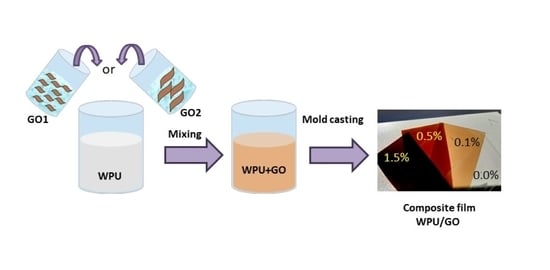
2.2. Film Preparation
2.3. Equipment
2.4. On Swelling Study
3. Results and discussion
3.1. Optical Photographs
3.2. Elemental Analysis
3.3. IR Spectroscopy
3.4. Thermogravimetric Analysis
3.5. Mechanical Properties
3.6. Water Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Itapu, B.; Jayatissa, A.H. A Review in Graphene/Polymer Composites. Chem. Sci. Int. J. 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Ramji, B.R.; Nagaral, M. A review on graphene reinforced polymer matrix composites. Mater. Today 2018, 5, 2419–2428. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A Review on the Production Methods and Applications of Graphene-Based Materials. Nanomaterials 2021, 11, 2414. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, J.; Fang, C.; Yu, R.; Lei, W.; He, X.; Zhang, C. Preparation and characterization of lysozyme@carbon nanotubes/waterborne polyurethane composite and the potential application in printing inks. Prog. Org. Coat. 2020, 142, 105600. [Google Scholar] [CrossRef]
- Yu, R.; Wang, Q.; Wang, W.; Xiao, Y.; Wang, Z.; Zhou, X.; Zhang, X.; Zhu, X.; Fang, C. Polyurethane/graphene oxide nanocomposite and its modified asphalt binder: Preparation, properties and molecular dynamics simulation. Mater. Des. 2021, 209, 109994. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Tian, S. Recent advances in functional polyurethane and its application in leather manufacture: A review. Polymers 2020, 12, 1996. [Google Scholar] [CrossRef]
- Alizadegan, F.; Mirabedini, S.M.; Pazokifard, S.; Moghadam, S.G.; Farnood, R. Improving self-healing performance of polyurethane coatings using PU microcapsules containing bulky-IPDI-BA and nano-clay. Prog. Org. Coat. 2018, 123, 350–361. [Google Scholar] [CrossRef]
- Pokharel, P.; Lee, D.S. Thermal and Mechanical Properties of Reduced Graphene Oxide/Polyurethane Nanocomposite. J. Nanosci. Nanotechnol. 2014, 14, 5718–5721. [Google Scholar] [CrossRef]
- Akram, N.; Saeed, M.; Usman, M.; Mansha, A.; Anjum, F.; Zia, K.M.; Mahmood, I.; Mumtaz, N.; Gul Khan, W. Influence of Graphene Oxide Contents on Mechanical Behavior of Polyurethane Composites Fabricated with Different Diisocyanates. Polymers 2021, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Sinh, L.H.; Luong, N.D.; Seppälä, J. Enhanced mechanical and thermal properties of polyurethane/functionalised graphene oxide composites by in situ polymerisation. Plast. Rubber Compos. 2019, 48, 466–476. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mishra, A.K. (Eds.) Eco-Friendly Waterborne Polyurethanes: Synthesis, Properties, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in Waterborne Polyurethane and Polyurethane-Urea Dispersions and Their Eco-friendly Derivatives: A Review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Agnol, L.D.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-curable waterborne polyurethane coatings: A state-of-the-art and recent advances review. Prog. Org. Coat. 2021, 154, 106156. [Google Scholar] [CrossRef]
- Yin, X.; Pang, H.; Luo, Y.; Zhang, B. Eco-friendly functional two-component flame-retardant waterborne polyurethane coatings: A review. Polym. Chem. 2021, 12, 5400–5411. [Google Scholar] [CrossRef]
- Bao, L.-H.; Lan, Y.-J.; Zhang, S.-F. Synthesis and properties of waterborne polyurethane dispersions with ions in the soft segments. J. Polym. Res. 2006, 13, 507–514. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Song, L.; Yang, H.; Hu, Y.; Yeoh, G.H. Fabrication and characterization of graphene-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method. Surf. Coat. Technol. 2012, 206, 4778–4784. [Google Scholar] [CrossRef]
- Kale, M.B.; Luo, Z.; Zhang, X.; Dhamodharan, D.; Divakaran, N.; Mubarak, S.; Wu, L.; Xu, Y. Waterborne polyurethane/graphene oxide-silica nanocomposites with improved mechanical and thermal properties for leather coatings using screen printing. Polymer 2019, 170, 43–53. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Kumar, B.; Strankowski, M.; Cardinaels, R.; Moldenaers, P.; Thomas, S.; Grohens, Y. Dielectric properties of modified graphene oxide filled polyurethane nanocomposites and its correlation with rheology. Compos. Sci. Technol. 2014, 104, 18–25. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, P.; Fan, H.; Sun, Z.; Wen, J. Covalently functionalized graphene towards molecular-level dispersed waterborne polyurethane nanocomposite with balanced comprehensive performance. Appl. Surf. Sci. 2019, 471, 595–606. [Google Scholar] [CrossRef]
- Larraza, I.; Alonso-Lerma, B.; Gonzalez, K.; Gabilondo, N.; Perez-Jimenez, R.; Corcuera, M.A.; Arbelaiz, A.; Eceiza, A. Waterborne polyurethane and graphene/graphene oxide-based nanocomposites: Reinforcement and electrical conductivity. Express Polym. Lett. 2020, 14, 1018–1033. [Google Scholar] [CrossRef]
- Jiang, Q.; Liao, X.; Li, J.; Chen, J.; Wang, G.; Yi, J.; Yang, Q.; Li, G. Flexible thermoplastic polyurethane/reduced graphene oxide composite foams for electromagnetic interference shielding with high absorption characteristic. Compos. Part A Appl. Sci. Manuf. 2019, 123, 310–319. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D. In situ reduction of graphene oxide in waterborne polyurethane matrix and the healing behavior of nanocomposites by multiple ways. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 202–209. [Google Scholar] [CrossRef]
- Romani, E.C.; Nardecchia, S.; Vilani, C.; Qi, S.; Dong, H.; Freire, F.L. Synthesis and characterization of polyurethane/reduced graphene oxide composite deposited on steel. J. Coat. Technol. Res. 2018, 15, 1371–1377. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Zaarei, D.; Gudarzi, M.M. Lightweight flexible polyurethane/reduced ultralarge graphene oxide composite foams for electromagnetic interference shielding. RSC Adv. 2016, 6, 27517–27527. [Google Scholar] [CrossRef]
- Tounici, A.; Martín-Martínez, J.M. Addition of small amounts of graphene oxide in the polyol for synthesizing waterborne polyurethane-urea adhesives with improved adhesion properties. Int. J. Adhes. Adhes. 2020, 104, 102725. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z. Preparation and characterization of a novel polyurethane/polyurethane modified graphene oxide composites. Colloid Polym. Sci. 2021, 299, 1767–1776. [Google Scholar] [CrossRef]
- Song, H.; Wang, M.; Wang, Y.; Zhang, Y.; Umar, A.; Guo, Z. Waterborne Polyurethane/Graphene Oxide Nanocomposites with Enhanced Properties. Sci. Adv. Mater. 2017, 9, 1895–1904. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Wang, S.; Jiang, C.; Xie, Y.; Yang, M.; Shi, H. A novel and feasible approach for polymer amine modified graphene oxide to improve water resistance, thermal, and mechanical ability of waterborne polyurethane. Appl. Surf. Sci. 2019, 491, 301–312. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Baskakov, S.A.; Baskakova, Y.V.; Volfkovich, Y.M.; Shulga, N.Y.; Skryleva, E.A.; Parkhomenko, Y.N.; Belay, K.G.; Gutsev, G.L.; Rychagov, A.Y.; et al. Supercapacitors with graphene oxide separators and reduced graphite oxide electrodes. J. Power Sources 2015, 279, 722–730. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acidmodified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash Reduction and Patterning of Graphite Oxide and Its Polymer Composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef]
- Karthika, P.; Rajalakshmi, N.; Dhathathreyan, K.S. Functionalized Exfoliated Graphene Oxide as Supercapacitor Electrodes. Soft Nanosci. Lett. 2012, 2, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Jiao, Q.; Zhao, Y.; Li, H. Vapor diffusion synthesis of CoFe2O4 hollow sphere/graphene composites as absorbing materials. J. Mater. Chem. A 2013, 2, 735–744. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D. Mechanical enhancement of self-healing waterborne polyurethane by graphene oxide. Prog. Org. Coat. 2018, 121, 73–79. [Google Scholar] [CrossRef]
- Ding, J.N.; Fan, Y.; Zhao, C.X.; Liu, Y.B.; Yu, C.T.; Yuan, N.Y. Electrical Conductivity of Waterborne Polyurethane/Graphene Composites Prepared by Solution Mixing. J. Compos. Mater. 2012, 46, 747–752. [Google Scholar] [CrossRef]
- Corish, P.J. Identification and Analysis of Polyurethane Rubbers by Infrared Spectroscopy. Anal. Chem. 1959, 31, 1298–1306. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, Ł.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. J. Spectrosc. 2016, 2016, 7520741. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Xu, X.; He, X.; Yan, Y. Preparation and Characterization of Graphene Oxide-Modified Sapium sebiferum Oil-Based Polyurethane Composites with Improved Thermal and Mechanical Properties. Polymers 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Xu, K.; Liu, H.; Cai, H.L.; Su, J.X.; Fu, Z.E.; Guo, Y.; Chen, M.C. Preparation and properties of waterborne polyurethanes with natural dimer fatty acids based polyester polyol as soft segment. Prog. Org. Coat. 2011, 72, 612–620. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Shin, C.M.; Kim, B.K. Morphological and physical properties of a thermoplastic polyurethane reinforced with functionalized graphene sheet. Polym. Int. 2009, 58, 412–417. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/Polyurethane Nanocomposites for Improved Gas Barrier and Electrical Conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Choi, J.T.; Kim, D.H.; Ryu, K.S.; Lee, H.; Jeong, H.M.; Shin, C.M.; Kim, J.H.; Kim, B.K. Functionalized graphene sheet/polyurethane nanocomposites: Effect of particle size on physical properties. Macromol. Res. 2011, 19, 809–814. [Google Scholar] [CrossRef]
- Cai, D.; Jin, J.; Yusoh, K.; Rafiq, R.; Song, M. High performance polyurethane/functionalized graphene nanocomposites with improved mechanical and thermal properties. Compos. Sci. Technol. 2012, 72, 702–707. [Google Scholar] [CrossRef]
- Bian, J.; Lin, H.L.; He, F.X.; Wei, X.W.; Chang, I.T.; Sancaktar, E. Fabrication of microwave exfoliated graphite oxide reinforced thermoplastic polyurethane nanocomposites: Effects of filler on morphology, mechanical, thermal and conductive properties. Compos. Part A Appl. Sci. Manuf. 2013, 47, 72–82. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, B.K. Infrared light actuated shape memory effects in crystalline polyurethane/graphene chemical hybrids. Smart Mater. Struct. 2014, 23, 025038. [Google Scholar] [CrossRef]
- Strankowski, M.; Piszczyk, Ł.; Kosmela, P.; Korzeniewski, P. Morphology and the physical and thermal properties of thermoplastic polyurethane reinforced with thermally reduced graphene oxide. Pol. J. Chem. Technol. 2015, 17, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Canales, J.; Muñoz, M.E.; Fernández, M.; Santamaría, A. Rheology, electrical conductivity and crystallinity of a polyurethane/graphene composite: Implications for its use as a hot-melt adhesive. Compos. Part A Appl. Sci. Manuf. 2016, 84, 9–16. [Google Scholar] [CrossRef]
- Strankowski, M.; Korzeniewski, P.; Strankowska, J.; Anu, A.S.; Thomas, S. Morphology, mechanical and thermal properties of thermoplastic polyurethane containing reduced graphene oxide and graphene nanoplatelets. Materials 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tounici, A.; Martín-Martínez, J.M. Addition of Graphene Oxide in Different Stages of the Synthesis of Waterborne Polyurethane-Urea Adhesives and Its Influence on Their Structure, Thermal, Viscoelastic and Adhesion Properties. Materials 2020, 13, 2899. [Google Scholar] [CrossRef] [PubMed]
- Han, X.B.; Gao, J.; Chen, Z.Y.; Tang, X.Q.; Zhao, Y.; Chen, T. Correlation between microstructure and properties of graphene oxide/waterborne polyurethane composites investigated by positron annihilation spectroscopy. RSC Adv. 2020, 10, 32436–32442. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Maiti, P. The effect of chemical tagging of graphene oxide in thermoplastic polyurethane on gelation behavior. Polymer 2022, 253, 124999. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. In situ polymerization of graphene nanosheets and polyurethane with enhanced mechanical and thermal properties. J. Mater. Chem. 2011, 21, 422. [Google Scholar] [CrossRef]




| Sample | C | O | H | N | S |
|---|---|---|---|---|---|
| GO1 | 45.28 | 49.70 | 2.72 | 0.00 | 2.30 |
| GO2 | 45.32 | 50.82 | 3.03 | 0.00 | 0.83 |
| WPU | 58.03 | 29.96 | 8.74 | 2.99 | 0.29 |
| WPU/GO1 (0.5 wt%) | 57.76 | 30.18 | 8.74 | 2.96 | 0.37 |
| WPU/GO1 (1.0 wt%) | 58.14 | 29.55 | 8.84 | 3.07 | 0.41 |
| WPU/GO1 (1.5 wt%) | 57.89 | 30.21 | 8.77 | 2.94 | 0.19 |
| GO Content | Young’s Modulus, MPa | * σb, MPa | ** εb, % | |||
|---|---|---|---|---|---|---|
| WPU/GO1 | WPU/GO2 | WPU/GO1 | WPU/GO2 | WPU/GO1 | WPU/GO2 | |
| 0% | 7.55 | 7.55 | 18.94 | 18.94 | 790 | 790 |
| 0.1% | 8.69 | 9.90 | 21.63 | 17.44 | 894 | 772 |
| 0.5% | 11.96 | 18.52 | 15.66 | 16.07 | 678 | 680 |
| 1% | 18.23 | 30.15 | 16.65 | 12.05 | 700 | 501 |
| 1.5% | 25.42 | - | 16.66 | - | 640 | - |
| 2% | 42.95 | - | 15.81 | - | 433 | - |
| Sample | SwD for WPU/GO1 | SwD for WPU/GO2 |
|---|---|---|
| Initial WPU | 27.52 ± 0.99 | 27.52 ± 0.99 |
| 0.1% GO | 25.69 ± 2.04 | 32.95 ± 1.16 |
| 0.5% GO | 23.84 ± 1.01 | 33.94 ± 2.31 |
| 1% GO | 21.91 ± 0.67 | 30.76 ± 0.91 |
| 1.5% GO | 23.19 ± 1.15 | - |
| 2% GO | 27.45 ± 1.38 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskakov, S.A.; Baskakova, Y.V.; Dvoretskaya, E.V.; Krasnikova, S.S.; Lesnichaya, V.A.; Shulga, Y.M.; Gutsev, G.L. Mechanical and Water Absorption Properties of Waterborne Polyurethane/Graphene Oxide Composites. Materials 2023, 16, 178. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16010178
Baskakov SA, Baskakova YV, Dvoretskaya EV, Krasnikova SS, Lesnichaya VA, Shulga YM, Gutsev GL. Mechanical and Water Absorption Properties of Waterborne Polyurethane/Graphene Oxide Composites. Materials. 2023; 16(1):178. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16010178
Chicago/Turabian StyleBaskakov, Sergey A., Yulia V. Baskakova, Elizaveta V. Dvoretskaya, Svetlana S. Krasnikova, Valentina A. Lesnichaya, Yury M. Shulga, and Gennady L. Gutsev. 2023. "Mechanical and Water Absorption Properties of Waterborne Polyurethane/Graphene Oxide Composites" Materials 16, no. 1: 178. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16010178