Tribological Properties of WS2 Thin Films Containing Graphite-like Carbon and Ni Interlayers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The Surface Morphology and Structure of the Films
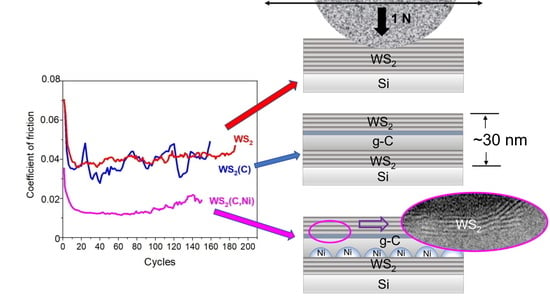
3.2. The Tribological Performance of the Obtained Films
3.3. Analysis of the Friction and Tribomodification of the Films
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spalvins, T. A review of resent advances in solid film lubrication. J. Vac. Sci. Technol. 1987, 5, 212–219. [Google Scholar] [CrossRef]
- Müller, C.; Menoud, C.; Maillat, M.; Hintermann, H.E. Thick compact MoS2 coatings. Surf. Coat. Technol. 1988, 36, 351–359. [Google Scholar] [CrossRef]
- Fominskii, V.; Markeev, A.; Nevolin, V. Pulsed ion beams for modification of metal surface properties. Vacuum 1991, 42, 73–74. [Google Scholar] [CrossRef]
- Pope, L.E.; Panitz, J.K.G. The effect of Hertzian stress and test atmosphere on the friction coefficient of MoS2 coatings. Surf. Coat. Technol. 1988, 36, 341–350. [Google Scholar] [CrossRef]
- Zhuang, W.; Fan, X.; Li, W.; Li, H.; Zhang, L.; Peng, J.; Cai, Z.; Mo, J.; Zhang, G.; Zhu, M. Comparing space adaptability of diamond-like carbon and molybdenum disulfide films toward synergistic lubrication. Carbon 2018, 134, 163–173. [Google Scholar] [CrossRef]
- Shi, J.; Ma, G.; Han, C.; Li, G.; Liu, Y.; Liu, Q. Tribological properties and bearing application of Mo-based films in space environment. Vacuum 2021, 188, 110217. [Google Scholar] [CrossRef]
- Trivedi, H.K.; Massey, M.M.; Bhattacharya, R.S.; Strahl, G.A.; Collumb, D. Next generation lubrication system for weapons. Tribol. Lett. 2001, 10, 229–235. [Google Scholar] [CrossRef]
- Behrens, B.A.; Maier, H.J.; Hübner, S.; Bonk, C.; Almohallami, A.; Lummer, C.; Schein, P.; Scheland, H.; Micke-Camuz, M. Wear Behavior of MoS2 Lubricant Layers during Sheet Metal Forming. Procedia Eng. 2017, 183, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Zhanga, X.; Vitchev, R.G.; Lauwerens, W.; Stals, L.; He, J.; Celis, J.-P. Effect of crystallographic orientation on fretting wear behavior of MoSx coatings in dry and humid air. Thin Solid Films 2001, 396, 69–77. [Google Scholar] [CrossRef]
- Fominski, V.; Demin, M.; Nevolin, V.; Fominski, D.; Romanov, R.; Gritskevich, M.; Smirnov, N. Reactive Pulsed Laser Deposition of Clustered-Type MoSx (x ~ 2, 3, and 4) Films and Their Solid Lubricant Properties at Low Temperature. Nanomaterials 2020, 10, 653. [Google Scholar] [CrossRef]
- Ren, S.; Shang, K.; Cui, M.; Wang, L.; Jibin, P.; Yi, P. Structural design of MoS2-based coatings toward high humidity and wide temperature. J. Mater. Sci. 2019, 54, 11889–11902. [Google Scholar] [CrossRef]
- Hu, J.J.; Zabinski, J.S.; Bultman, J.E.; Sanders, J.H.; Voevodin, A.A. Structure characterization of pulsed laser deposited MoSx–WSey composite films of tribological interests. Tribol. Lett. 2006, 24, 126–135. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Grigoriev, S.N.; Gnedovets, A.G.; Romanov, R.I. Pulsed laser deposition of composite Mo-Se-Ni-C coatings using standard and shadow mask configuration. Surf. Coat. Technol. 2012, 206, 5046–5054. [Google Scholar] [CrossRef]
- Cavaleiro, A.; Polcar, T. Review on self-lubricant transition metal dichalcogenide nanocomposite coatings alloyed with carbon. Surf. Coat. Technol. 2011, 206, 686–695. [Google Scholar] [CrossRef]
- Hudeca, T.; Mikula, M.; Satrapinskyy, L.; Roch, T.; Truchlý, M.; Švec, P., Jr.; Huminiuc, T.; Polcard, T. Structure, mechanical and tribological properties of Mo-S-N solid lubricant coatings. Appl. Surf. Sci. 2019, 486, 1–14. [Google Scholar] [CrossRef]
- Yaqub, T.B.; Vuchkov, T.; Sanguino, P.; Polcar, T.; Cavaleiro, A. Comparative study of DC and RF sputtered MoSe2 coatings containing carbon—An approach to optimize stoichiometry, microstructure, crystallinity, and hardness. Coatings 2020, 10, 133. [Google Scholar] [CrossRef] [Green Version]
- Maharana, H.S.; Mondal, K. Manifestation of Hall–Petch breakdown in nanocrystalline electrodeposited Ni-MoS2 coating and its structure dependent wear resistance behavior. Surf. Coat. Technol. 2021, 410, 126950. [Google Scholar] [CrossRef]
- Gao, X.; Fu, Y.; Jiang, D.; Wang, D.; Xu, S.; Liu, W.; Weng, L.; Yang, J.; Sun, J.; Hu, M. Constructing WS2/MoS2 nano-scale multilayer film and understanding its positive response to space environment. Surf. Coat. Technol. 2018, 353, 8–17. [Google Scholar] [CrossRef]
- Noshiro, J.; Watanabe, S.; Sakurai, T.; Miyake, S. Friction properties of co-sputtered sulfide/DLC solid lubricating films. Surf. Coat. Technol. 2006, 200, 5849–5854. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Ji, L.; Ye, Y.; Chen, J.; Zhou, H. A long-lifetime MoS2/a-C:H nanoscale multilayer film with extremely low internal stress. Surf. Coat. Technol. 2013, 236, 438–443. [Google Scholar] [CrossRef]
- Xu, J.; He, T.F.; Chai, L.Q.; Qiao, L.; Wang, P.; Liu, W.M. Growth and characteristics of self-assembled MoS2/Mo-S-C nanoperiod multilayers for enhanced tribological performance. Sci. Rep. 2016, 6, 25378. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.M.; Donnet, C.; Mogne, T.L.; Epicier, T. Superlubricity of molybdenum disulphide. Phys. Rev. B 1993, 48, 10583. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Verhoeven, G.S.; Pradeep, N.; Frenken, J.W.M.; Heimberg, J.A.; Zandbergen, H.W. Superlubricity of Graphite. Phys. Rev. Lett. 2004, 92, 126101. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Shi, S.; Ma, M.; Zheng, Q. Rotational Instability in Superlubric Joints. Phys. Rev. Lett. 2019, 122, 246101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, D.; Narayanan, B.; Cherukara, M.J.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Zinovev, A.; Sumant, A.V. Operando tribochemical formation of onion-like-carbon leads to macroscale superlubricity. Nat. Commun. 2018, 9, 1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Yang, X.; Pu, J.; He, Z.; Xiong, L. Extraordinary macroscale lubricity of sonication-assisted fabrication of MoS2 nano-ball and investigation of in situ formation mechanism of graphene induced by tribochemical reactions. Appl. Surf. Sci. 2020, 510, 145456. [Google Scholar] [CrossRef]
- Yu, G.; Gong, Z.; Jiang, B.; Wang, D.; Bai, C.; Zhang, J. Superlubricity for hydrogenated diamond like carbon induced by thin MoS2 and DLC layer in moist air. Diam. Relat. Mater. 2020, 102, 107668. [Google Scholar] [CrossRef]
- Chen, X.; Yin, X.; Qi, W.; Zhang, C.; Choi, J.; Wu, S.; Wang, R.; Luo, J. Atomic-scale insights into the interfacial instability of superlubricity in hydrogenated amorphous carbon films. Sci. Adv. 2020, 6, eaay1272. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Ji, X.; Ma, W.; Zhang, B.; Zhang, J. Hierarchical structure graphitic-like/MoS2 film as superlubricity material. Appl. Surf. Sci. 2017, 413, 381–386. [Google Scholar] [CrossRef]
- Jiang, A.; Cao, X.; Wang, Z.; Ma, J.; Xiao, J.; Ma, S. Friction performance and corrosion resistance of MoS2/DLC composite films deposited by magnetron sputtering. Results Phys. 2021, 25, 104278. [Google Scholar] [CrossRef]
- Gao, K.; Lai, Z.; Jia, Q.; Zhang, B.; Wei, X.; Zhang, J. Bilayer a-C:H/MoS2 film to realize superlubricity in open atmosphere. Diam. Relat. Mater. 2020, 108, 107973. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Z.; Tian, P.; Gong, Z.; Zhang, J. Macro-scale superlow friction enabled when MoS2 flakes lubricate hydrogenated diamond-like carbon film. Ceram. Int. 2021, 47, 10980–10989. [Google Scholar] [CrossRef]
- Nossa, A.; Cavaleiro, A.; Carvalho, N.J.M.; Kooi, B.J.; De Hosson, J.T.M. On the microstructure of tungsten disulfide films alloyed with carbon and nitrogen. Thin Solid Films 2005, 484, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-N.; Wang, C.-B.; Wang, H.-D.; Xu, B.-S.; Zhuang, D.-M.; Liu, J.-J.; Li, G.-L. Microstructure and tribological properties of WS2/MoS2 multilayer films. Appl. Surf. Sci. 2012, 258, 1944–1948. [Google Scholar] [CrossRef]
- Xu, S.; Gao, X.; Hu, M.; Sun, J.; Jiang, D.; Zhou, F.; Liu, W.; Weng, L. Nanostructured WS2–Ni composite films for improved oxidation, resistance and tribological performance. Appl. Surf. Sci. 2014, 288, 15–25. [Google Scholar] [CrossRef]
- Gao, X.; Fu, Y.; Jiang, D.; Wang, D.; Yang, J.; Weng, L.; Hu, M.; Sun, J. Structural, Mechanical, and Tribological Properties of WS2-Al Nanocomposite Film for Space Application. Tribol. Lett. 2018, 66, 137. [Google Scholar] [CrossRef]
- Cao, H.; Momand, J.; Syari’ati, A.; Wen, F.; Rudolf, P.; Xiao, P.; De Hosson, J.T.M.; Pei, Y. Temperature-Adaptive Ultralubricity of a WS2/a-C Nanocomposite Coating: Performance from Room Temperature up to 500 °C. ACS Appl. Mater. Interfaces 2021, 13, 28843–28854. [Google Scholar] [CrossRef]
- Watanabe, S.; Noshiro, J.; Miyake, S. Tribological characteristics of WS2/MoS2 solid lubricating multilayer films. Surf. Coat. Technol. 2004, 183, 347–351. [Google Scholar] [CrossRef]
- Watanabe, S.; Noshiro, J.; Miyake, S. Friction properties of WS2/MoS2 multilayer films under vacuum environment. Surf. Coat. Technol. 2004, 188–189, 644–648. [Google Scholar] [CrossRef]
- Xi, J.; Huang, X.; Hu, M.; Xiang, W. Dependence of laser parameters on structural properties of pulsed laser-deposited MoS2 thin films applicable for field effect transistors. J. Mater. Sci. Mater. Electron. 2020, 31, 21118–21127. [Google Scholar] [CrossRef]
- Seo, S.; Kim, S.; Choi, H.; Lee, J.; Yoon, H.; Piao, G.; Park, J.-C.; Jung, Y.; Song, J.; Jeong, S.Y.; et al. Direct in situ growth of centimeter-scale multi-heterojunction MoS2/WS2/WSe2 thin-film catalyst for photo-electrochemical hydrogen evolution. Adv. Sci. 2019, 6, 1900301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fominski, V.Y.; Romanov, R.I.; Nevolin, V.N.; Fominski, D.V.; Komleva, O.V.; Popov, V.V. Formation of ultrathin MoS2 films using laser-based methods. J. Phys. Conf. Ser. 2019, 1238, 012007. [Google Scholar] [CrossRef] [Green Version]
- Ossia, P.M.; Bottania, C.E.; Miotello, A. Pulsed-laser deposition of carbon: From DLC to cluster-assembled films. Thin Solid Films 2005, 482, 2–8. [Google Scholar] [CrossRef]
- Fominski, V.; Nevolin, V.; Romanov, R.; Smirnov, A.; Scharff, W. Atomic mixing and chemical bond formation in MoSx/Fe thin-film system deposited from a laser plume in a high-intensity electrostatic field. Thin Solid Films 2002, 422, 39–47. [Google Scholar] [CrossRef]
- Boubiche, N.; Hamouchi, J.E.; Hulik, J.; Abdesslam, M.; Speisser, C.; Djeffal, F.; Normand, F.L. Kinetics of graphitization of thin diamond-like carbon (DLC) films catalyzed by transition metal. Diam. Relat. Mater. 2019, 91, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Berman, D.; Deshmukh, S.A.; Narayanan, B.; Sankaranarayanan, S.K.R.S.; Yan, Z.; Balandin, A.A.; Zinovev, A.; Rosenmann, D.; Sumant, A.V. Metal-induced rapid transformation of diamond into single and multilayer graphene on wafer scale. Nat. Commun. 2016, 7, 12099. [Google Scholar] [CrossRef] [Green Version]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Su, W.; Chen, F.; Fu, L.; Ding, S.; Song, K.; Huang, X.; Zhang, L. Atypical defect-mediated photoluminescence and resonance Raman spectroscopy of monolayer WS2. J. Phys. Chem. C 2019, 123, 3900–3907. [Google Scholar] [CrossRef]
- Markeev, A.M.; Kozodaev, M.G.; Slavich, A.S.; Romanov, R.I.; Zarubin, S.S. Influence of reducing agent on properties of thin WS2 nanosheets prepared by sulfurization of atomic layer-deposited WO3. J. Phys. Chem. C 2020, 124, 28169–28177. [Google Scholar] [CrossRef]
- Romanov, R.I.; Kozodaev, M.G.; Chernikova, A.G.; Zabrosaev, I.V.; Chouprik, A.A.; Zarubin, S.S.; Novikov, S.M.; Volkov, V.S.; Markeev, A.M. Thickness-Dependent Structural and Electrical Properties of WS2 Nanosheets Obtained via the ALD-Grown WO3 Sulfurization Technique as a Channel Material for Field-Effect Transistors. ACS Omega 2021, 6, 34429–34437. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. A 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 2003, 67, 155306. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Origin of the 1150 cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 2001, 63, 121405. [Google Scholar] [CrossRef]
- Sun, L.; Gao, K.; Jia, Q.; Bai, C.; Zhang, B.; Tan, X.; Zhang, J. Grown of superlubricity a-C:H/MoS2 film on 9Cr18Mo steel for industrial application. Diam. Relat. Mater. 2021, 117, 108479. [Google Scholar] [CrossRef]
- Ungár, T.; Gubicza, J.; Trichy, G.; Pantea, C.; Zerda, T.W. Size and shape of crystallites and internal stresses in carbon blacks. Compos. Part A Appl. Sci. Manuf. 2005, 36, 431–436. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhemelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascon, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Fominski, V.; Fominski, D.; Romanov, R.; Gritskevich, M.; Demin, M.; Shvets, P.; Maksimova, K.; Goikhman, A. Specific features of reactive pulsed laser deposition of solid lubricating nanocomposite Mo-S-C-H thin-film coatings. Nanomaterials 2020, 10, 2456. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Qin, Z.; Zeng, D.; Zhang, J.; Wu, C.; Wen, Y.; Shan, B.; Xie, C. Effect of layer number on recovery rate of WS2 nanosheets for ammonia detection at room temperature. Appl. Surf. Sci. 2017, 414, 244–250. [Google Scholar] [CrossRef]
- Wu, H.; Li, W.; Li, W.; Dai, Y.; Guo, J.; Wang, S.; Song, J.; Odunmbaku, G.O.; Zhang, H.; Boi, F.S. Evidence for increased metallicity arising from carbon-sulfur bonding and amorphization effects in sulfur-doped pyrolytic graphite. Diam. Relat. Mater. 2022, 121, 108729. [Google Scholar] [CrossRef]
- Odunmbakua, O.; Songa, J.; Wang, S.; Taallaha, A.; Dai, Y.; Li, W.; Li, W.; He, Y.; Guo, J.; Zhanga, H.; et al. Nucleation of carbon-sulfur phases by manipulation of vertically-aligned mm-long films of iron-filled few-wall/multiwall carbon nanotubes. Carbon Trends 2021, 5, 100102. [Google Scholar] [CrossRef]
- Bautista-Flores, C.; Arellano-Peraza, J.S.; Sato-Berrú, R.Y.; Camps, E.; Mendoza, D. Sulfur and few-layer graphene interaction under thermal treatments. Chem. Phys. Lett. 2016, 665, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Yang, Z.; Sun, L.; Gao, K.; Zhang, B.; Zhang, X.; Zhang, J. Catalytic superlubricity via in-situ formation of graphene during sliding friction on Au@a-C:H films. Carbon 2022, 186, 180–192. [Google Scholar] [CrossRef]
- Sundberg, J.; Nyberg, H.; Särhammar, E.; Nyberg, T.; Jacobson, S.; Jansson, U. Influence of composition, structure and testing atmosphere on the tribological performance of W–S–N coatings. Surf. Coat. Technol. 2014, 285, 86–94. [Google Scholar] [CrossRef]
- Nossa, A.; Cavaleiro, A. The influence of the addition of C and N on the wear behaviour of W-S-C/N coatings. Surf. Coat. Technol. 2001, 142–144, 984–991. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanov, R.I.; Fominski, D.V.; Demin, M.V.; Gritskevich, M.D.; Doroshina, N.V.; Volkov, V.S.; Fominski, V.Y. Tribological Properties of WS2 Thin Films Containing Graphite-like Carbon and Ni Interlayers. Materials 2023, 16, 282. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16010282
Romanov RI, Fominski DV, Demin MV, Gritskevich MD, Doroshina NV, Volkov VS, Fominski VY. Tribological Properties of WS2 Thin Films Containing Graphite-like Carbon and Ni Interlayers. Materials. 2023; 16(1):282. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16010282
Chicago/Turabian StyleRomanov, Roman I., Dmitry V. Fominski, Maxim V. Demin, Mariya D. Gritskevich, Natalia V. Doroshina, Valentyn S. Volkov, and Vyacheslav Yu. Fominski. 2023. "Tribological Properties of WS2 Thin Films Containing Graphite-like Carbon and Ni Interlayers" Materials 16, no. 1: 282. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16010282