Zn-doped Mono- and Biphasic Calcium Phosphate Materials Derived from Agriculture Waste and Their Potential Biomedical Applications: Part I
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Synthesis Procedure
2.2. The Obtained Materials’ Characterization
2.3. Antibacterial Activity Studies
2.3.1. Strains and Maintenance
2.3.2. Direct Contact Test
2.4. Cytotoxicity Assessment In Vitro
2.5. Statistical Analysis
3. Results
3.1. ATR-FTIR Characterization of Undoped and Zn-Doped Powders
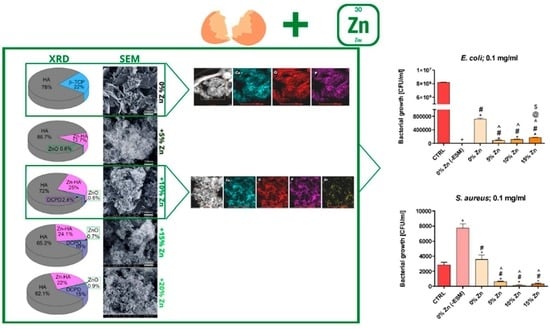
3.2. XRD, SEM, TEM and Porous Structure Characterization of Undoped and Zn-Doped Powders
3.3. ICP-OES Determination of Zn Content in the Doped Powders
3.4. XPS Characterization of Undoped and 10% Zn-Doped Material
3.5. Antibacterial Activity of Undoped and Zn-Doped Materials
3.6. Cytotoxicity Assessment In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragab, H.S.; Ibrahim, F.A.; Abdallah, F.; Al-Ghamdi, A.A.; El-Tantawy, F.; Radwan, N.; Yakuphanoglu, F. Synthesis and In Vitro Antibacterial Properties of Hydroxyapatite Nanoparticles. J. Pharm. Biol. Sci. 2014, 9, 77–85. [Google Scholar] [CrossRef]
- Pajor, K.; Michalicha, A.; Belcarz, A.; Pajchel, L.; Zgadzaj, A.; Wojas, F.; Kolmas, J. Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes. Int. J. Mol. Sci. 2022, 23, 7102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, Y.; Chen, Z.; Lin, S.; Yuan, Z.; Ren, X.; et al. One-Pot Synthesis and Characterization of Zn-Doped Hydroxyapatite Nanocomposites. Mater. Chem. Phys. 2017, 199, 122–130. [Google Scholar] [CrossRef]
- Kaygili, O.; Tatar, C. The Investigation of Some Physical Properties and Microstructure of Zn-Doped Hydroxyapatite Bioceramics Prepared by Sol–Gel Method. J. Sol-Gel Sci. Technol. 2012, 61, 296–309. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-Substituted Hydroxyapatite: A Biomaterial with Enhanced Bioactivity and Antibacterial Properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of Hydroxyapatite and Carbonated Apatite by Photo Acoustic FTIR Spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Lee, D.; Kumta, P.N. Chemical Synthesis and Stabilization of Magnesium Substituted Brushite. Mater. Sci. Eng. C 2010, 30, 934–943. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and Characterization of Zn-Doped Hydroxyapatite: Scaffold Application, Antibacterial and Bioactivity Studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [Green Version]
- Nenen, A.; Maureira, M.; Neira, M.; Orellana, S.L.; Covarrubias, C.; Moreno-Villoslada, I. Synthesis of Antibacterial Silver and Zinc Doped Nano-Hydroxyapatite with Potential in Bone Tissue Engineering Applications. Ceram. Int. 2022, 48, 34750–34759. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangir, M.U.; Islam, F.; Wong, S.Y.; Jahan, R.A.; Matin, M.A.; Li, X.; Arafat, M.T. Comparative Analysis and Antibacterial Properties of Thermally Sintered Apatites with Varied Processing Conditions. J. Am. Ceram. Soc. 2021, 104, 1023–1039. [Google Scholar] [CrossRef]
- Marques, C.F.; Olhero, S.; Abrantes, J.C.C.; Marote, A.; Ferreira, S.; Vieira, S.I.; Ferreira, J.M.F. Biocompatibility and Antimicrobial Activity of Biphasic Calcium Phosphate Powders Doped with Metal Ions for Regenerative Medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Szcześ, A.; Sternik, D. The Preparation of Calcium Phosphate Adsorbent from Natural Calcium Resource and Its Application for Copper Ion Removal. Environ. Sci. Pollut. Res. 2021, 28, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Kalbarczyk, M.; Szcześ, A.; Kantor, I.; May, Z.; Sternik, D. Synthesis and Characterization of Calcium Phosphate Materials Derived from Eggshells from Different Poultry with and without the Eggshell Membrane. Materials 2022, 15, 934. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-Doped Hydroxyapatite Coatings and Their Antibacterial Efficacy against Escherichia Coli and Staphylococcus Aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier Transform Infrared Spectroscopy of Hydroxyapatite Co-Doped with Zinc and Fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Sridevi, S.; Ramya, S.; Akshaikumar, K.; Kavitha, L.; Manoravi, P.; Gopi, D. Fabrication of Zinc Substituted Hydroxyapatite/Cellulose Nano Crystals Biocomposite from Biowaste Materials for Biomedical Applications. Mater. Today Proc. 2020, 26, 3583–3587. [Google Scholar] [CrossRef]
- Adeogun, A.I.; Ofudje, A.E.; Idowu, M.A.; Kareem, S.O. Facile Development of Nano Size Calcium Hydroxyapatite Based Ceramic from Eggshells: Synthesis and Characterization. Waste Biomass Valorization 2018, 9, 1469–1473. [Google Scholar] [CrossRef]
- Valarmathi, N.; Sumathi, S. Zinc Substituted Hydroxyapatite/Silk Fiber/Methylcellulose Nanocomposite for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 214, 324–337. [Google Scholar] [CrossRef]
- Peñaflor Galindo, T.G.; Kataoka, T.; Fujii, S.; Okuda, M.; Tagaya, M. Preparation of Nanocrystalline Zinc-Substituted Hydroxyapatite Films and Their Biological Properties. Colloid Interface Sci. Commun. 2016, 10–11, 15–19. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed Laser Deposition of Copper and Zinc Doped Hydroxyapatite Coatings for Biomedical Applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic Calcium Phosphate Ceramics for Bone Reconstruction: A Review of Biological Response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Cheng, Y.; Ma, L.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Bao, H.; Li, X.; et al. Nanosized Strontium Substituted Hydroxyapatite Prepared from Egg Shell for Enhanced Biological Properties. J. Biomater. Appl. 2018, 32, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, W.; Cho, J.H.; Oh, N.S.; Lee, M.H.; Lee, S.J. Comparison of Bone Formation in Rabbits Using Hydroxyapatite and β-Tricalcium Phosphate Scaffolds Fabricated From Egg Shells. Adv. Mater. Res. 2008, 47–50, 999–1002. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative Study of Portland Cement Hydration by X-Ray Diffraction/Rietveld Analysis and Independent Methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Condon, J.B. Chapter 1-An Overview of Physisorption. In Surface Area and Porosity Determinations by Physisorption; Condon, J.B., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 1–27. ISBN 978-0-444-51964-1. [Google Scholar]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. Infrared Spectrosc.-Mater. Sci. Eng. Technol. 2012, 123–148. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Lorenzo, L.M. Studies on Calcium Deficient Apatites Structure by Means of MAS-NMR Spectroscopy. J. Mater. Sci. Mater. Med. 2005, 16, 393–398. [Google Scholar] [CrossRef]
- Massit, A.; Elyacoubi, A.; Rezzouk, A.; Chafik, B.; Chafik El Idrissi, B. Thermal Behavior of Mg-Doped Calcium-Deficient Apatite and Stabilization of β Tricalcium Phosphate. Biointerface Res. Appl. Chem. 2020, 10, 6837–6845. [Google Scholar] [CrossRef]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [Green Version]
- Battistoni, C.; Casaletto, M.P.; Ingo, G.M.; Kaciulis, S.; Mattogno, G.; Pandolfi, L. Surface Characterization of Biocompatible Hydroxyapatite Coatings. Surf. Interface Anal. 2000, 29, 773–781. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” Cytotoxicity of Ions-Adsorbing Hydroxyapatite—Corrected Method of Cytotoxicity Evaluation for Ceramics of High Specific Surface Area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Reis, R.L. Bilayered Chitosan-Based Scaffolds for Osteochondral Tissue Engineering: Influence of Hydroxyapatite on in Vitro Cytotoxicity and Dynamic Bioactivity Studies in a Specific Double-Chamber Bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavsson, J.; Ginebra, M.P.; Engel, E.; Planell, J. Ion Reactivity of Calcium-Deficient Hydroxyapatite in Standard Cell Culture Media. Acta Biomater. 2011, 7, 4242–4252. [Google Scholar] [CrossRef]







| 0% Zn | 5% Zn | 10% Zn | 15% Zn | 20% Zn | |
|---|---|---|---|---|---|
| Specific surface area BET [m2/g] | 36.3 ± 0.2 | 80.1 ± 0.6 | 56.0 ± 0.3 | 30.0 ± 0.2 | 25.2 ± 1.0 |
| Total pore volume [cm3/g] | 0.187 | 0.553 | 0.352 | 0.242 | 0.179 |
| Micropore volume [cm3/g] | 0.005 | 19.719 | 0.005 | 0.004 | 0.002 |
| Average pore width (BET) [nm] | 20.6 | 27.3 | 25.1 | 32.2 | 28.1 |
| Pore diameter from the adsorption BJH [nm] | 25.4 | 34.6 | 29.4 | 38.0 | 32.2 |
| Sample | Zn Composition, mol % | (Ca + Zn)/P Molar Ratio | |
|---|---|---|---|
| Planned | Experimental | Experimental | |
| 0% Zn | 0 | 0 | 1.50 ± 0.04 |
| 5% Zn | 5 | 5.4 ± 0.3 | 1.45 ± 0.03 |
| 10% Zn | 10 | 10.8 ± 0.6 | 1.42 ± 0.01 |
| 15% Zn | 15 | 15.6 ± 0.9 | 1.34 ± 0.02 |
| 20% Zn | 20 | 18.6 ± 1.0 | 1.42 ± 0.06 |
| PS Control | 0%Zn (−ESM) | 0% Zn | 5% Zn | 10% Zn | 15% Zn | |
|---|---|---|---|---|---|---|
| Cell viability [% of PS control] | 100 ± 7.0 | 35.5 ± 3.9 * | 6.4 ± 1.9 *,# | 47.6 ± 8.2 *,#,^ | 32.7 ± 7.9 *,^,@ | 7.3 ± 2.1 *,#,@,$ |
| Ca2+ concentration in sample extracts [μg/mL] | 68.5 ± 4.8 | 57.0± 3.9 | 33.7 ± 5.3 *,# | 52.0 ± 1.2 *,^ | 58.9 ± 1.7 ^ | 55.6 ± 8.1 ^ |
| concentration in sample extracts [μg/mL] | 43.1 ± 2.8 | 145.1 ± 1.1 * | 179.2 ± 4.2 *,# | 166.2 ± 2.6 *,#,^ | 163.2 ± 4.7 *,#,^ | 170.7 ± 0.6 *,#,^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalbarczyk, M.; Szcześ, A.; Belcarz, A.; Kazimierczak, P.; May, Z. Zn-doped Mono- and Biphasic Calcium Phosphate Materials Derived from Agriculture Waste and Their Potential Biomedical Applications: Part I. Materials 2023, 16, 1971. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16051971
Kalbarczyk M, Szcześ A, Belcarz A, Kazimierczak P, May Z. Zn-doped Mono- and Biphasic Calcium Phosphate Materials Derived from Agriculture Waste and Their Potential Biomedical Applications: Part I. Materials. 2023; 16(5):1971. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16051971
Chicago/Turabian StyleKalbarczyk, Marta, Aleksandra Szcześ, Anna Belcarz, Paulina Kazimierczak, and Zoltan May. 2023. "Zn-doped Mono- and Biphasic Calcium Phosphate Materials Derived from Agriculture Waste and Their Potential Biomedical Applications: Part I" Materials 16, no. 5: 1971. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16051971