Are We Ready for NGS HIV Drug Resistance Testing? The Second “Winnipeg Consensus” Symposium
Abstract
:1. HIV Drug Resistance Is a Global Challenge
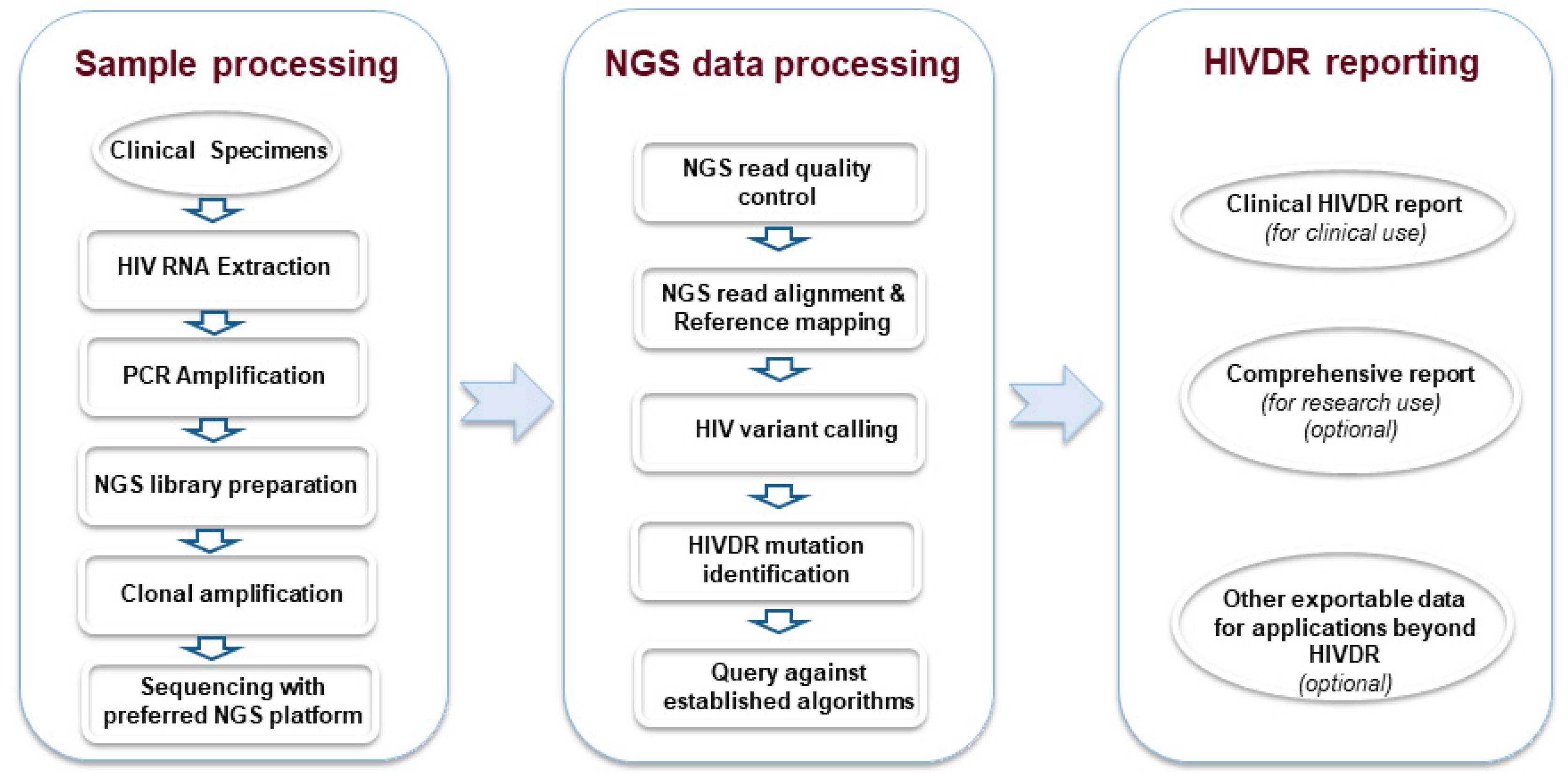
2. Next-Generation Sequencing (NGS) Is an Emerging New Standard for HIVDR Testing
3. Standardization of NGS HIVDR Testing Is Urgently Required
4. Initiation of an International Symposium Series on NGS HIVDR Testing
5. Are We Ready for NGS HIV Drug Resistance Testing? The Second “Winnipeg Consensus” Symposium
6. Remaining Challenges for Generalized Implementation of NGS HIVDR Testing
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AIDSinfo. FDA-Approved HIV Medicines. 2020. Available online: https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-approved-hiv-medicines (accessed on 30 January 2020).
- National Institute of Allergy and Infectious Diseases. Drugs That Fight HIV-1: A Reference Guide for Prescription HIV-1 Medications; The U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Yombi, J.C.; Mertes, H. Treatment as prevention for HIV infection: Current data, challenges, and global perspectives. AIDS Rev. 2018, 20, 131–140. [Google Scholar] [CrossRef]
- Menendez-Arias, L. Targeting HIV: Antiretroviral therapy and development of drug resistance. Trends Pharm. Sci. 2002, 23, 381–388. [Google Scholar] [CrossRef]
- Coffin, J.M. HIV population dynamics in vivo: Implications for genetic variation, pathogenesis, and therapy. Science 1995, 267, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.D. Perspectives series: Host/pathogen interactions. Dynamics of HIV-1 replication in vivo. J. Clin. Investig. 1997, 99, 2565–2567. [Google Scholar] [CrossRef]
- Domingo, E.; Sheldon, J.; Perales, C. Viral quasispecies evolution. Microbiol. Mol. Biol. Rev. 2012, 76, 159–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clutter, D.S.; Jordan, M.R.; Bertagnolio, S.; Shafer, R.W. HIV-1 drug resistance and resistance testing. Infect. Genet. Evol. 2016, 46, 292–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Action Plan on HIV Drug Resistance 2017–2021; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Hyle, E.P.; Scott, J.A.; Sax, P.E.; Millham, L.R.I.; Dugdale, C.M.; Weinstein, M.C.; Freedberg, K.A.; Walensky, R.P. Clinical impact and cost-effectiveness of genotype testing at human immunodeficiency virus diagnosis in the United States. Clin. Infect. Dis. 2020, 70, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.N.; Cambiano, V.; Nakagawa, F.; Magubu, T.; Miners, A.; Ford, D.; Pillay, D.; De Luca, A.; Lundgren, J.; Revill, P. Cost-Effectiveness of HIV Drug Resistance Testing to Inform Switching to Second Line Antiretroviral Therapy in Low Income Settings. PLoS ONE 2014, 9, e109148. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services (DHHS). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV 2017. Available online: https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003510.pdf (accessed on 26 May 2020).
- Günthard, H.F.; Calvez, V.; Paredes, R.; Pillay, D.; Shafer, R.W.; Wensing, A.M.; Jacobsen, D.M.; Richman, U.D. Human Immunodeficiency Virus Drug Resistance: 2018 Recommendations of the International Antiviral Society–USA Panel. Clin. Infect. Dis. 2018, 68, 177–187. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, A.-M.; Camacho, R.; Ceccherini-Silberstein, F.; De Luca, A.; Palmisano, L.; Paraskevis, D.; Paredes, R.; Poljak, M.; Schmit, J.-C.; Soriano, V.; et al. European recommendations for the clinical use of HIV drug resistance testing: 2011 update. Aids Rev. 2011, 13, 77–108. [Google Scholar] [PubMed]
- Simen, B.B.; Simons, J.F.; Hullsiek, K.H.; Novak, R.M.; MacArthur, R.D.; Baxter, J.D.; Huang, C.; Lubeski, C.; Turenchalk, G.S.; Braverman, M.S.; et al. Low-Abundance Drug-Resistant Viral Variants in Chronically HIV-Infected, Antiretroviral Treatment–Naive Patients Significantly Impact Treatment Outcomes. J. Infect. Dis. 2009, 199, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Korn, K.; Reil, H.; Walter, H.; Schmidt, B. Quality Control Trial for Human Immunodeficiency Virus Type 1 Drug Resistance Testing Using Clinical Samples Reveals Problems with Detecting Minority Species and Interpretation of Test Results. J. Clin. Microbiol. 2003, 41, 3559–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.A.; Li, J.-F.; Wei, X.; Lipscomb, J.; Irlbeck, D.; Craig, C.; Smith, A.; E Bennett, D.; Monsour, M.; Sandstrom, P.; et al. Minority HIV-1 Drug Resistance Mutations Are Present in Antiretroviral Treatment–Naïve Populations and Associate with Reduced Treatment Efficacy. PLoS Med. 2008, 5, e158. [Google Scholar] [CrossRef]
- Ji, H.; Li, Y.; Graham, M.R.; Liang, B.; Pilon, R.; Tyson, S.; Peters, G.; Tyler, S.; Merks, H.; Bertagnolio, S.; et al. Next-generation sequencing of dried blood spot specimens: A novel approach to HIV drug-resistance surveillance. Antivir. Ther. 2011, 16, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.; Lee, E.R.; Nykoluk, M.; Enns, E.; Liang, B.; Capina, R.; Gauthier, M.-K.; Domselaar, G.V.; Sandstrom, P.; Brooks, J.; et al. A MiSeq-HyDRA platform for enhanced HIV drug resistance genotyping and surveillance. Sci. Rep. 2019, 9, 8970. [Google Scholar] [CrossRef] [Green Version]
- Margulies, M.; Egholm, M.; Altman, W.E.; Attiya, S.; Bader, J.S.; Bemben, L.A.; Berka, J.; Braverman, M.S.; Chen, Y.-J.; Chen, Z.; et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005, 437, 376–380. [Google Scholar] [CrossRef]
- Hui, P. Next Generation Sequencing: Chemistry, Technology and Applications. Chem. Diagn. 2012, 336, 1–18. [Google Scholar]
- Bushman, F.D.; Hoffmann, C.; Ronen, K.; Malani, N.; Minkah, N.; Rose, H.M.; Tebas, P.; Wang, G.P. Massively parallel pyrosequencing in HIV research. AIDS 2008, 22, 1411–1415. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Mitsuya, Y.; Gharizadeh, B.; Ronaghi, M.; Shafer, R.W. Characterization of mutation spectra with ultra-deep pyrosequencing: Application to HIV-1 drug resistance. Genome Res. 2007, 17, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Minkah, N.; Leipzig, J.; Wang, G.; Arens, M.Q.; Tebas, P.; Bushman, F.D. DNA bar coding and pyrosequencing to identify rare HIV drug resistance mutations. Nucleic Acids Res. 2007, 35, e91. [Google Scholar] [CrossRef] [Green Version]
- Derache, A.; Iwuji, C.C.; Danaviah, S.; Giandhari, J.; Marcelin, A.-G.; Calvez, V.; De Oliveira, T.; Dabis, F.; Pillay, D.; Gupta, R.K. Predicted antiviral activity of tenofovir versus abacavir in combination with a cytosine analogue and the integrase inhibitor dolutegravir in HIV-1-infected South African patients initiating or failing first-line ART. J. Antimicrob. Chemother. 2018, 74, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekici, H.; Rao, S.D.; Sönnerborg, A.; Ramprasad, V.L.; Gupta, R.; Neogi, U. Cost-efficient HIV-1 drug resistance surveillance using multiplexed high-throughput amplicon sequencing: Implications for use in low- and middle-income countries. J. Antimicrob. Chemother. 2014, 69, 3349–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.M.; Meyer, A.M.; Winner, D.; Archer, J.; Feyertag, F.; Ruiz-Mateos, E.; Leal, M.; Robertson, D.L.; Schmotzer, C.L.; Quiñones-Mateu, M.E. Sensitive Deep-Sequencing-Based HIV-1 Genotyping Assay To Simultaneously Determine Susceptibility to Protease, Reverse Transcriptase, Integrase, and Maturation Inhibitors, as Well as HIV-1 Coreceptor Tropism. Antimicrob. Agents Chemother. 2014, 58, 2167–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Massé, N.; Tyler, S.; Liang, B.; Li, Y.; Merks, H.; Graham, M.; Sandstrom, P.; Brooks, J. HIV Drug Resistance Surveillance Using Pooled Pyrosequencing. PLoS ONE 2010, 5, e9263. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Li, Y.; Liang, B.; Pilon, R.; MacPherson, P.; Bergeron, M.; Kim, J.; Graham, M.; Van Domselaar, G.; Sandstrom, P.; et al. Pyrosequencing Dried Blood Spots Reveals Differences in HIV Drug Resistance between Treatment Naïve and Experienced Patients. PLoS ONE 2013, 8, e56170. [Google Scholar] [CrossRef]
- Keys, J.R.; Zhou, S.; Anderson, J.A.; Eron, J.J.; Rackoff, L.A.; Jabara, C.; Swanstrom, R. Primer ID Informs Next-Generation Sequencing Platforms and Reveals Preexisting Drug Resistance Mutations in the HIV-1 Reverse Transcriptase Coding Domain. AIDS Res. Hum. Retrovir. 2015, 31, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, H.R.; Dong, W.; Lee, G.Q.; Bangsberg, D.R.; Martin, J.N.; Mocello, A.R.; Boum, Y.; Karakas, A.; Kirkby, D.; Poon, A.F.Y.; et al. HIV Drug Resistance Testing by High-Multiplex “Wide” Sequencing on the MiSeq Instrument. Antimicrob. Agents Chemother. 2015, 59, 6824–6833. [Google Scholar] [CrossRef] [Green Version]
- Moscona, R.; Ram, D.; Wax, M.; Bucris, E.; Levy, I.; Mendelson, E.; Mor, O. Comparison between next-generation and Sanger-based sequencing for the detection of transmitted drug-resistance mutations among recently infected HIV-1 patients in Israel, 2000–2014. J. Int. AIDS Soc. 2017, 20, 21846. [Google Scholar] [CrossRef]
- Raymond, S.; Nicot, F.; Carcenac, R.; Lefebvre, C.; Jeanne, N.; Sauné, K.; Delobel, P.; Izopet, J. HIV-1 genotypic resistance testing using the Vela automated next-generation sequencing platform. J. Antimicrob. Chemother. 2018, 73, 1152–1157. [Google Scholar] [CrossRef]
- Tzou, P.L.; Ariyaratne, P.; Varghese, V.; Lee, C.; Rakhmanaliev, E.; Villy, C.; Yee, M.; Tan, K.; Michel, G.; Pinsky, B.A.; et al. Comparison of an In Vitro Diagnostic Next-Generation Sequencing Assay with Sanger Sequencing for HIV-1 Genotypic Resistance Testing. J. Clin. Microbiol. 2018, 56, e00105–e00118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Z.; Paredes, R.; Ribaudo, H.J.; Svarovskaia, E.S.; Metzner, K.J.; Kozal, M.; Hullsiek, K.H.; Balduin, M.; Jakobsen, M.R.; Geretti, A.M.; et al. Low-Frequency HIV-1 Drug Resistance Mutations and Risk of NNRTI-Based Antiretroviral Treatment Failure. JAMA 2011, 305, 1327–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzaule, S.C.; Ondoa, P.; Peter, T.; Mugyenyi, P.N.; Stevens, W.S.; De Wit, T.F.R.; Hamers, R.L. Affordable HIV drug-resistance testing for monitoring of antiretroviral therapy in sub-Saharan Africa. Lancet Infect. Dis. 2016, 16, e267–e275. [Google Scholar] [CrossRef]
- Telele, N.F.; Kalu, A.; Gebre-Selassie, S.; Fekade, D.; Abdurahman, S.; Marrone, G.; Neogi, U.; Tegbaru, B.; Sönnerborg, A. Pretreatment drug resistance in a large countrywide Ethiopian HIV-1C cohort: A comparison of Sanger and high-throughput sequencing. Sci. Rep. 2018, 8, 7556. [Google Scholar] [CrossRef]
- Alves, B.M.; Siqueira, J.; Prellwitz, I.M.; Botelho, O.M.; Da Hora, V.P.; Sanabani, S.; Recordon-Pinson, P.; Fleury, H.; Soares, E.A.; Soares, M.A. Estimating HIV-1 Genetic Diversity in Brazil Through Next-Generation Sequencing. Front. Microbiol. 2019, 10, 749. [Google Scholar] [CrossRef]
- Casadellà, M.; Paredes, R. Deep sequencing for HIV-1 clinical management. Virus Res. 2017, 239, 69–81. [Google Scholar] [CrossRef]
- FDA. FDA Authorizes Marketing of First Next-Generation Sequencing Test for Detecting HIV-1 Drug Resistance Mutations; FDA: White Oak, MD, USA, 2019.
- Raymond, S.; Nicot, F.; Abravanel, F.; Minier, L.; Carcenac, R.; Lefebvre, C.; Harter, A.; Martin-Blondel, G.; Delobel, P.; Izopet, J. Performance evaluation of the Vela Dx Sentosa next-generation sequencing system for HIV-1 DNA genotypic resistance. J. Clin. Virol. 2020, 122, 104229. [Google Scholar] [CrossRef]
- Weber, J.; Volkova, I.; Sahoo, M.K.; Tzou, P.L.; Shafer, R.W.; Pinsky, B.A. Prospective Evaluation of the Vela Diagnostics Next-Generation Sequencing Platform for HIV-1 Genotypic Resistance Testing. J. Mol. Diagn. 2019, 21, 961–970. [Google Scholar] [CrossRef]
- WHO. The Use of Next-Generation Sequencing Technologies for the Detection of Mutations Associated with Drug Resistance in Mycobacterium Tuberculosis Complex: Technical Guide; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ji, H.; Enns, E.; Brumme, C.; Parkin, N.; Howison, M.; Lee, E.R.; Capina, R.; Marinier, E.; Avila-Rios, S.; Sandstrom, P.; et al. Bioinformatic data processing pipelines in support of next-generation sequencing-based HIV drug resistance testing: The Winnipeg Consensus. J. Int. AIDS Soc. 2018, 21, e25193. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Julian, M.; Edgil, D.; Harrigan, P.R.; Sandstrom, P.; Godfrey, C.; Paredes, R. Next-Generation Human Immunodeficiency Virus Sequencing for Patient Management and Drug Resistance Surveillance. J. Infect. Dis. 2017, 216, S829–S833. [Google Scholar] [CrossRef]
- Howison, M.; Coetzer, M.; Kantor, R. Measurement error and variant-calling in deep Illumina sequencing of HIV. Bioinformatics 2018, 35, 2029–2035. [Google Scholar] [CrossRef]
- Lee, E.R.; Parkin, N.; Jennings, C.; Brumme, C.J.; Enns, E.; Casadellà, M.; Howison, M.; Coetzer, M.; Avila-Rios, S.; Capina, R.; et al. Performance comparison of next generation sequencing analysis pipelines for HIV-1 drug resistance testing. Sci. Rep. 2020, 10, 1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsall, D.; Golubchik, T.; De Cesare, M.; Limbada, M.; Kosloff, B.; MacIntyre-Cockett, G.; Hall, M.; Wymant, C.; Ansari, M.A.; Abeler-Dorner, L.; et al. A comprehensive genomics solution for HIV surveillance and clinical monitoring in a global health setting. BioRxiv 2018, 397083. [Google Scholar] [CrossRef]
- Jabara, C.B.; Jones, C.D.; Roach, J.; Anderson, J.A.; Swanstrom, R. Accurate sampling and deep sequencing of the HIV-1 protease gene using a primer ID. Proc. Natl. Acad Sci. USA 2011, 108, 20166–20171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Jones, C.; Mieczkowski, P.; Swanstrom, R. Primer ID Validates Template Sampling Depth and Greatly Reduces the Error Rate of Next-Generation Sequencing of HIV-1 Genomic RNA Populations. J. Virol. 2015, 89, 8540–8555. [Google Scholar] [CrossRef] [Green Version]
- Parkin, N.; Zaccaro, D.; Avila-Rios, S.; Brumme, C.; Hunt, G.; Ji, H.; Kantor, R.; Mbisa, J.L.; Predes, R.; Rivera-Amill, V.; et al. Multi-Laboratory comparison of next-generation to Sanger-based sequencing for HIV-1 drug resistance genotyping. In Proceedings of the XXVII International HIV Drug Resistance and Treatment Strategies Workshop, Johannesburg, South Africa, 22–23 October 2018. [Google Scholar]
- Lee, E.R.; Parkin, N.; Enns, E.; Brumme, C.J.; Casadella, M.; Howison, M.; Avila Rios, S.; Jennings, R.; Capina, R.; Marinier, E.; et al. Characterization and data assessment of next generation sequencing-based genotyping using existing HIV-1 drug resistance proficiency panels. In Proceedings of the XXVII International HIV Drug Resistance and Treatment Strategies Workshop, Johannesburg, South Africa, 22–23 October 2018. [Google Scholar]
- Ji, H.; Parkin, N.; Gao, F.; Denny, T.; Jennings, C.; Sandstrom, P.; Kantor, R. External Quality Assessment Program for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Logistical Considerations. Viruses 2020, 12, 556. [Google Scholar] [CrossRef]
- Lee, E.R.; Gao, F.; Sandstrom, P.; Ji, H. External Quality Assessment for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Unique Requirements and Challenges. Viruses 2020, 12, 550. [Google Scholar] [CrossRef]
- Eliseev, A.; Gibson, K.M.; Avdeyev, P.; Novik, D.; Bendall, M.L.; Perez-Losada, M.; Alexeev, N.; Crandall, K.A. Evaluation of haplotype callers for next-generation sequencing of viruses. Infect Genet. Evol. 2020, 82, 104277. [Google Scholar] [CrossRef]
- Kasibhatla, S.M.; Waman, V.P.; Kale, M.; Kulkarni-Kale, U. Analysis of Next-Generation Sequencing Data in Virology-Opportunities and Challenges. In Next Generation Sequencing—Advances, Applications and Challenges; eBook (PDF); Jerzy, K., Ed.; IntechOpen: London, UK, 2016; ISBN 978-953-51-5419-8. [Google Scholar] [CrossRef]
- Marinier, E.; Enns, E.; Tran, C.; Fogel, M.; Peters, C.; Kidwai, A.; Ji, H.; Van Domselaar, G. quasitools: A Collection of Tools for Viral Quasispecies Analysis. BioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Kozal, M.; Chiarella, J.; John, E.P.S.; Moreno, E.A.; Simen, B.B.; E Arnold, T.; Lataillade, M. Prevalence of low-level HIV-1 variants with reverse transcriptase mutation K65R and the effect of antiretroviral drug exposure on variant levels. Antivir. Ther. 2011, 16, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Goodman, D.D.; Zhou, Y.; Margot, N.; McColl, D.J.; Zhong, L.; Borroto-Esoda, K.; Miller, M.D.; Svarovskaia, E. Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS 2011, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- A Mbunkah, H.; Bertagnolio, S.; Hamers, R.L.; Hunt, G.M.; Inzaule, S.; De Wit, T.F.R.; Paredes, R.; Parkin, N.T.; Jordan, M.R.; Metzner, K.J.; et al. Low-Abundance Drug-Resistant HIV-1 Variants in Antiretroviral Drug-Naive Individuals: A Systematic Review of Detection Methods, Prevalence, and Clinical Impact. J. Infect. Dis. 2019, 221, 1584–1597. [Google Scholar] [CrossRef] [PubMed]
| Generation of Sequencing Technology a | Manufacturer | Sequencing Mechanism | Error Rates (%) | NGS Platform | Maximum Read Length (Bases) b | Data Throughput (Gigabases/Run) | Estimated Instrument Cost (USD) |
|---|---|---|---|---|---|---|---|
| 2nd generation | Illumina | Sequencing-by-Synthesis | ~0.1 | iSeq | 2 × 150 | 0.3–1.2 | 19,900 |
| MiniSeq | 2 × 150 | 1.7–7.5 | 50,000 | ||||
| MiSeq | 2 × 300 | 0.3–15 | 100,000 | ||||
| NextSeq | 2 × 150 | 10–120 | 250,000 | ||||
| HiSeq | 2 × 150 | 10–1000 | 650,000 | ||||
| Thermo Fisher | Ion semiconductor sequencing | ~1 | PGM | 400 | 0.08–2.0 | 80,000 | |
| S5 | 400 | 0.6–1.5 | 60,000 | ||||
| Proton | 200 | 10–15 | 149,000 | ||||
| Vela Diagnostics | Ion semiconductor sequencing | ~1 | Sentosa SQ301 | 200 | 0.6–2.0 | 400,000 | |
| 3rd generation | Pacific Biosciences | Single-molecule real-time sequencing (no PCR involved) | ~13 | PacBio RSII | 60,000 | 0.5–1.0 | 750,000 |
| Sequel | 60,000 | 5–10 | 350,000 | ||||
| Oxford Nanopore Technologies | Single-molecule real-time sequencing (no DNA synthesis involved) | ~12 | MinIon | 100,000+ | 10–20 | 1000 | |
| GridIon | 100,000+ | 50–100 | 2400 | ||||
| PromethIon | 100,000+ | 480–960 | 25,000 |
| Challenges | Operational Needs and Current Status | Steps Moving Forward |
|---|---|---|
| Lack of appropriate reference materials for EQA and PT |
|
|
| Lack of protocols that work consistently, without sampling bias, for different HIV-1 subtypes, specimen formats or VLs |
|
|
| Lack of simplified and cost-effective assays suitable for resource-limited settings and/or point-of-care use |
|
|
| Lack of unified assay validation and internal quality control (IQC) strategies |
|
|
| Lack of effective EQA strategies that enable objective laboratory performance assessment |
|
|
| Short NGS reads that hinder quasispecies reconstruction and downstream cluster analysis |
|
|
| Tools for improved bioinformatic data processing and HIVDR interpretation |
|
|
| The clinical relevance of NGS-identified MRVs remains to be better defined |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Sandstrom, P.; Paredes, R.; Harrigan, P.R.; Brumme, C.J.; Avila Rios, S.; Noguera-Julian, M.; Parkin, N.; Kantor, R. Are We Ready for NGS HIV Drug Resistance Testing? The Second “Winnipeg Consensus” Symposium. Viruses 2020, 12, 586. https://0-doi-org.brum.beds.ac.uk/10.3390/v12060586
Ji H, Sandstrom P, Paredes R, Harrigan PR, Brumme CJ, Avila Rios S, Noguera-Julian M, Parkin N, Kantor R. Are We Ready for NGS HIV Drug Resistance Testing? The Second “Winnipeg Consensus” Symposium. Viruses. 2020; 12(6):586. https://0-doi-org.brum.beds.ac.uk/10.3390/v12060586
Chicago/Turabian StyleJi, Hezhao, Paul Sandstrom, Roger Paredes, P. Richard Harrigan, Chanson J. Brumme, Santiago Avila Rios, Marc Noguera-Julian, Neil Parkin, and Rami Kantor. 2020. "Are We Ready for NGS HIV Drug Resistance Testing? The Second “Winnipeg Consensus” Symposium" Viruses 12, no. 6: 586. https://0-doi-org.brum.beds.ac.uk/10.3390/v12060586





