In Silico Insights into the SARS CoV-2 Main Protease Suggest NADH Endogenous Defences in the Control of the Pandemic Coronavirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Structure-Based Studies
2.1.1. Ligand Preparation
2.1.2. Protein Preparation
2.1.3. Docking Validation
2.1.4. Induced Fit Docking
2.2. Biotarget Finder Module (DRUDIT)
3. Results and Discussion
3.1. Repurposing of Known HIV Protease Inhibitors
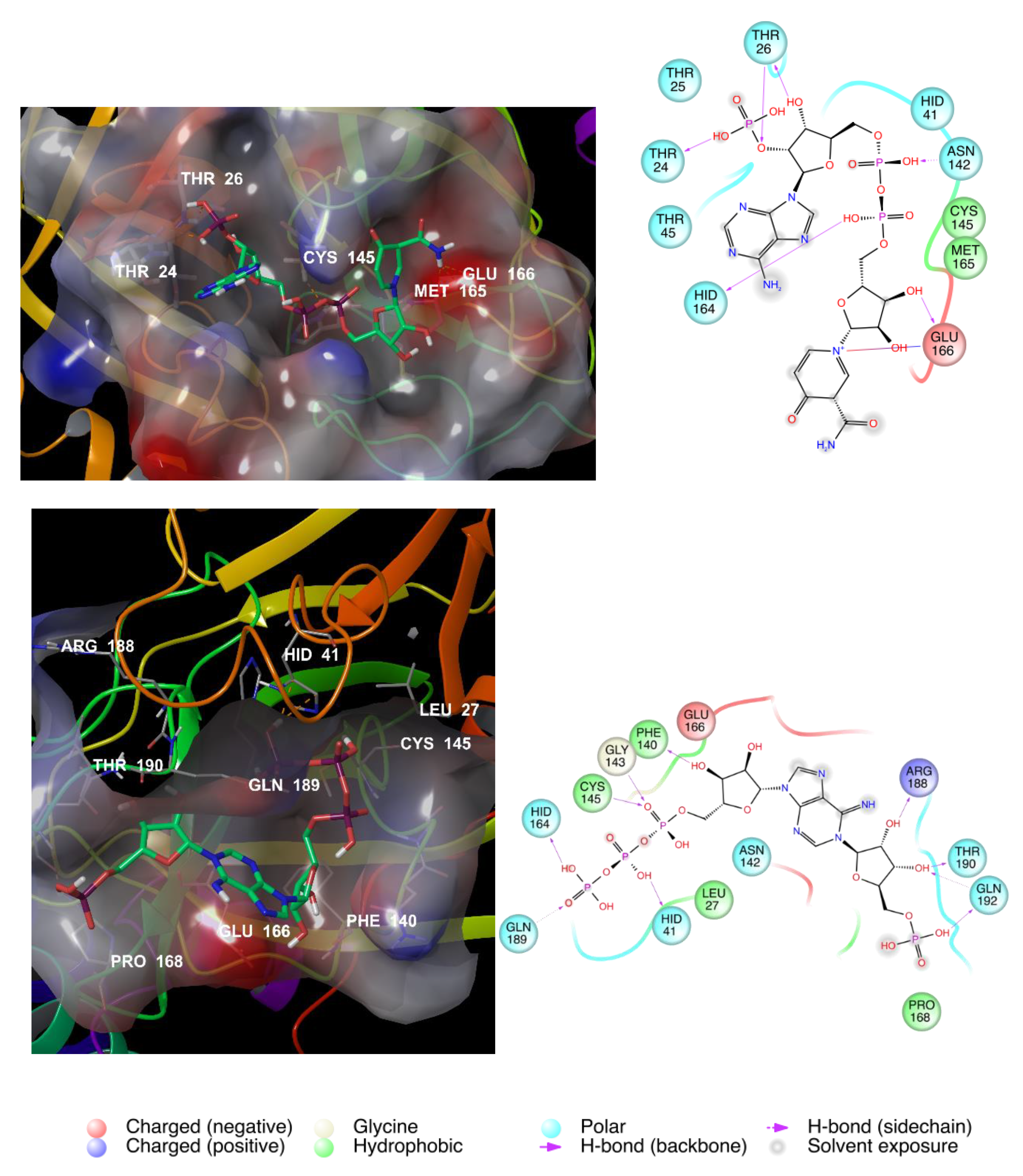
3.2. NAD as a Potential Modulator of COVID-19 MPRO
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, P.; Yang, X.; Wang, X.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupferschmidt, K. Cohen Mar J WHO launches global megatrial of the four most promising coronavirus treatments. Science 2020, 22. [Google Scholar] [CrossRef]
- Martorana, A.; Perricone, U.; Lauria, A. The Repurposing of Old Drugs or Unsuccessful Lead Compounds by in Silico Approaches: New Advances and Perspectives. Curr Top Med Chem. 2016, 16, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, W.; Zhao, X.; Zai, J.; Li, X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020, 92, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 2020, 22, 244. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, B.; Jin, Z.; Yang, H.; Rao, Z. The crystal structure of COVID-19 main protease in complex with an inhibitor N3. Protein DataBank 2020. [Google Scholar] [CrossRef]
- Zhenming, J.; Xiaoyu, D.; Yechun, X.; Yongqiang, D.; Meiqin, L.; Yao, Z.; Bing, Z.; Xiaofeng, L.; Leike, Z.; Chao, P.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Schrödinger Release 2017–2, LigPrep; Schrödinger, LLC: New York, NY, USA, 2017.
- Schrödinger Suite 2017-2 Protein Preparation Wizard; Epik, Schrödinger, LLC: New York, NY, USA, 2017.
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren TAMainz, D.T.; Maple, J.R.; Murphy, R.; et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shaw, D.E.; Shelley, M.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an Induced Fit Receptor Structure in Virtual Screening. Chem. Biol. Drug Design. 2006, 67, 83–84. [Google Scholar] [CrossRef]
- Maestro, version 10.2, Schrödinger; LLC: New York, NY, USA, 2017.
- Zhong, H.; Tran, L.M.; Stang, J.L. Induced-fit docking studies of the active and inactive states of protein tyrosine kinases. J. Mol. Graph. Model. 2009, 28, 336–346. [Google Scholar] [CrossRef]
- Wanga, H.; Aslanian, R.; Madison, V.S. Induced-fit docking of mometasone furoate and further evidence for glucocorticoid receptor 17α pocket flexibility. J. Mol. Graph. Model. 2008, 27, 512–521. [Google Scholar] [CrossRef]
- Luo, H.J.; Wang, J.Z.; Deng, W.Q.; Zou, K. Induced-fit docking and binding free energy calculation on furostanol saponins from Tupistra chinensis as epidermal growth factor recep-tor inhibitors. Med. Chem. Res. 2013, 22, 4970–4979. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A Hierarchical Approach to All-Atom Protein Loop Prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of Crystal Packing Forces in Determining Protein Sidechain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Lauria, A.; Mannino, S.; Gentile, C.; Mannino, G.; Martorana, A.; Peri, D. DRUDIT: Web-based DRUgs DIscovery Tools to design small molecules as modulators of biological targets. Bioinformatics 2020, 36, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Ippolito, M.; Almerico, A.M. Principal component analysis on molecular descriptors as an alternative point of view in the search of new Hsp90 inhibitors. Comput. Biol. Chem. 2009, 33, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Ippolito, M.; Almerico, A.M. Combined use of PCA and QSAR/QSPR to predict the drugs mechanism of action. An application to the NCI ACAM database. QSAR Comb. Sci. 2009, 28, 387–395. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [Green Version]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Nakagawa, T. NAD metabolism: Implications in aging and longevity. Ageing Res. Rev. 2018, 47, 1–17. [Google Scholar] [CrossRef]
- To, K.F.; Lo, A.W. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): The tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J. Pathol. 2004, 203, 740–743. [Google Scholar] [CrossRef] [Green Version]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Korteweg, C. Pathology and Pathogenesis of Severe Acute Respiratory Syndrome. Am. J. Pathol. 2007, 170, 1136–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, N.J.; Gutteridge, J.M.; Baker, C.; Evans, T.W.; Quinlan, G.J. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: Evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit. Care Med. 1999, 27, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Luboeinski, T.; Markart, P.; Ruppert, C.; Daum, C.; Grimminger, F.; Seeger, W.; Günther, A. Alveolar antioxidant status in patients with acute respiratory distress syndrome. Eur. Respir. J. 2004, 24, 994–999. [Google Scholar] [CrossRef] [PubMed]






| Z | D | G | ||
|---|---|---|---|---|
| a | b | c | ||
| 50 | 200 | 0.79 | 0.66 | 0.56 |
| 500 | 0.72 | 0.54 | 0.47 | |
| 1000 | 0.47 | 0.37 | 0.47 | |
| 100 | 200 | 0.85 | 0.77 | 0.69 |
| 500 | 0.75 | 0.59 | 0.52 | |
| 1000 | 0.59 | 0.45 | 0.39 | |
| ID | Prime Score | XP Docking Score | IFD Score | SARS CoV−2 Mpro DAS |
|---|---|---|---|---|
| 3730 | −10,682 | −15.09 | −549.2 | 0.583 |
| 5884 | −11,035 | −13.05 | −564.8 | 0.91 |
| 5885 | −10,967 | −13.15 | −561.5 | 0.917 |
| 16500 | −10,813 | −13.66 | −554.3 | 0.89 |
| 23700 | −10,937 | −15.89 | −562.7 | 0.863 |
| 123926 | −10,797 | −12.10 | −551.9 | 0.973 |
| 163884 | −10,976 | −11.52 | −560.3 | 0.957 |
| 165491 | −10,851 | −13.16 | −555.7 | 0.983 |
| 170119 | −10,720 | −12.63 | −548.6 | 0.917 |
| 183797 | −10,712 | −10.74 | −546.3 | 0.843 |
| 445888 | −10,770 | −14.55 | −553.0 | 0.867 |
| 446724 | −10,803 | −14.81 | −554.9 | 0.897 |
| 447657 | −10,740 | −11.19 | −548.2 | 0.863 |
| 448108 | −11,130 | −13.91 | −570.4 | 0.817 |
| 448209 | −10,825 | −14.53 | −555.8 | 0.9 |
| 449129 | −10,746 | −13.41 | −550.7 | 0.807 |
| 449366 | −10,687 | −13.08 | −547.4 | 0.877 |
| 4369128 | −10,898 | −11.97 | −556.9 | 0.9 |
| 5281793 | −10,709 | −15.15 | −550.6 | 0.893 |
| 5288989 | −10,974 | −12.10 | −560.8 | 0.857 |
| 5289104 | −10,805 | −12.48 | −552.8 | 0.99 |
| 5289382 | −11,615 | −12.13 | −592.9 | 0.967 |
| 5289437 | −116,960 | −11.65 | −596.4 | 0.897 |
| 6323200 | −11,664 | −12.84 | −596.0 | 0.723 |
| 9875516 | −11,712 | −12.69 | −598.3 | 0.733 |
| 16019963 | −11,660 | −16.08 | −599.1 | 0.767 |
| 17754101 | −11,663 | −13.05 | −596.2 | 0.94 |
| 49867432 | −11,777 | −13.52 | −602.4 | 0.893 |
| Drug | SARS CoV-2 Mpro (DAS) | HIV-1 Protease (DAS) |
|---|---|---|
| Amprenavir | 0.836 | 0.538 |
| Asunaprevir | 0.446 | 0.696 |
| Darunavir | 0.841 | 0.844 |
| Fosamprenavir | 0.868 | 0.763 |
| Indinavir | 0.463 | 0.901 |
| JE-2147 | 0.784 | 0.88 |
| L-756423 | 0.444 | 0.89 |
| Lopinavir | 0.457 | 0.910 |
| Nelfinavir | 0.506 | 0.907 |
| Ritonavir | 0.463 | 0.881 |
| Saquinavir | 0.475 | 0.898 |
| Tipranavir | 0.818 | 0.756 |
| Drug | XP Docking Score | Prime Score | IFD Score |
|---|---|---|---|
| Fosamprenavir | −10.80 | −117,070 | −596.2 |
| Darunavir | −9.45 | −11,631 | −591.1 |
| Tipranavir | −8.32 | −11,615 | −589.1 |
| Amprenavir | −10.48 | −11,601 | −590.5 |
| Age | 0–1 | 30–50 | 51–70 | >71 |
|---|---|---|---|---|
| NAD (ng NAD/mg protein) mean ± SEM | 8.54 ± 1.55 | 2.74 ± 0.41 | 1.06 ± 0.15 | 1.08 ± 0.19 |
| # of COVID-19 deaths (in %) | 0 | 3 | 11 | 86 |
| cpd | XP Docking Score | Prime Score | IFD Score | SARS CoV−2 Mpro (DAS) |
|---|---|---|---|---|
| NAD+ | −13.15 | −11,628 | −594.6 | 0.98 |
| NADH | −12.40 | −11,682 | −596.5 | 0.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martorana, A.; Gentile, C.; Lauria, A. In Silico Insights into the SARS CoV-2 Main Protease Suggest NADH Endogenous Defences in the Control of the Pandemic Coronavirus Infection. Viruses 2020, 12, 805. https://0-doi-org.brum.beds.ac.uk/10.3390/v12080805
Martorana A, Gentile C, Lauria A. In Silico Insights into the SARS CoV-2 Main Protease Suggest NADH Endogenous Defences in the Control of the Pandemic Coronavirus Infection. Viruses. 2020; 12(8):805. https://0-doi-org.brum.beds.ac.uk/10.3390/v12080805
Chicago/Turabian StyleMartorana, Annamaria, Carla Gentile, and Antonino Lauria. 2020. "In Silico Insights into the SARS CoV-2 Main Protease Suggest NADH Endogenous Defences in the Control of the Pandemic Coronavirus Infection" Viruses 12, no. 8: 805. https://0-doi-org.brum.beds.ac.uk/10.3390/v12080805





