1. Introduction
Over a century after the first successful vaccination of a human [
1], rabies is still widely spread around the globe, claiming an estimated 60.000 human lives each year [
2]. Due to its inevitably fatal course and the wide array of susceptible host species, the virus is considered to be one of the most, if not the most important viral zoonotic disease worldwide [
3,
4,
5]. Dogs are the main reservoir species, responsible for 99% of human cases [
6]. While canine rabies remains under control in most developed areas of the world due to ubiquitous vaccination regiments, among other things [
7,
8], these methods may not be feasible in many countries where the disease is still endemic. In addition to the difficulty of reaching free-roaming sub-populations of dogs, financial and social constraints often do not allow for employment of the necessary staff and equipment to execute mass vaccination campaigns via parenteral route [
9,
10]. According to the World Health Organization (WHO) and the World Organization for Animal Health (OIE), a minimum of 70% of the dog population in a specific area needs to be immunized against rabies to achieve a lasting protective herd immunity [
11]. Compared to vaccination campaigns focusing solely on parenteral immunization, the use of oral vaccination baits has been shown to be more effective in terms of cost per dog vaccinated and apparent coverage achieved [
10,
12]. Oral rabies vaccination (ORV) in dogs has the potential to fulfil the criteria set by both the WHO and the OIE for control and eventual elimination of dog-mediated rabies [
12,
13]. This fact, plus the aforementioned financial incentive, makes ORV in dogs a promising method to achieve the goal of global elimination of dog-mediated human rabies deaths by 2030 [
2].
The concept of ORV is based on 3 components: the vaccine, the bait, and the distribution system. Bait uptake is mainly monitored by the detection of a marker substance such as tetracycline incorporated into the bait matrix. Upon bait consumption, this marker is taken up by the animal and deposited in bone and teeth, where it can later be detected via fluorescence microscopy [
14]. In addition to tetracycline, other markers have been used to assess bait ingestion, including various types of iophenoxic acids (which can be detected in blood samples), as well as clenbuterol and rhodamine B (which are detectable in both fur and whiskers) [
15,
16,
17,
18]. However, the vaccine bait should not only be highly attractive to the targeted species, but also ensure prompt release of its fluid contents into the oral cavity. If an animal swallows the bait without perforating the incorporated sachet, the vaccine will not come into contact with the tonsils or oral mucous membranes, where an immune response may be triggered [
19]. Therefore, not all individuals that accept and consume a vaccine bait can automatically be considered vaccinated [
20]. In other cases, animals may attempt to separate the bait matrix from the sachet, or rough handling of the bait may occur, both of which result in sometimes significant spillage and loss of inoculant [
19]. To address these concerns and further optimize ORV delivery methods, bait studies should not only assess bait acceptance but also evaluate bait handling in detail.
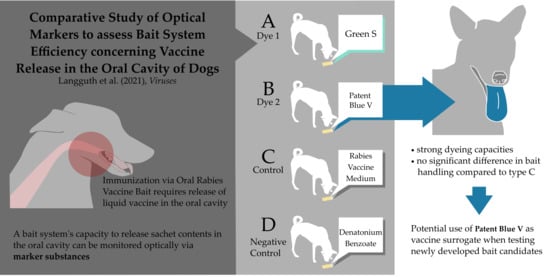
This requires a reliable method for documenting the efficient release of the sachet contents in the oral cavity. The use of marker substances like iophenoxic acid, tetracycline, or rhodamine B, incorporated into the bait matrix, would not suffice, as these can also be taken up in the intestine. Thus, it is not possible to determine how and where exactly these substances were absorbed, should samples test positive. Although rhodamine B has been used for detection in the oral cavity [
21], it is not readily visible from a distance. For some species, including free-roaming dogs, which are largely inaccessible, inspection of the oral cavity at close range is often not feasible. Consequently, this study’s aim was the selection of a suitable marker substance, used as a surrogate for the vaccine, which allows a reliable evaluation of a vaccine bait system’s capacity to deliver liquid contents into the oral cavity, without handling the animal. Previous bait acceptance and uptake studies using a dye incorporated in the sachet found that it was extremely difficult to observe the coloration of an animal’s tongue and oral mucous membranes without direct observation, which required restraint [
22,
23,
24]. The results of our study support the idea of using a more intense colorant, such as Patent Blue V, at the appropriate concentration, to ascertain release of sachet contents into the oral cavity of an animal that has been offered an oral rabies vaccine bait, without restraining it.
4. Discussion
Oral rabies vaccination has been shown to be a powerful tool to control and ultimately eliminate rabies from targeted reservoir species in different settings around the world [
32,
33,
34,
35]. It is suggested that ORV can contribute significantly to the elimination of dog-mediated rabies in areas with a high number of animals inaccessible for parenteral vaccination [
36]. One of the pre-requisites for ORV is a suitable bait. Many bait studies have been conducted all over the world to evaluate bait palatability and acceptance in dogs [
23,
24,
37,
38,
39,
40,
41,
42], but only few have directly addressed vaccine release in the oral cavity [
22].
This study gives detailed insights into the handling of a highly attractive bait by dogs. The use of oral color indicators in ORV baits, as surrogates of live oral rabies vaccines, provides the ability to determine successful release of sachet contents and thus allows evaluation of the efficacy of newly developed bait candidates.
In this study, the dog’s response to baits containing the medium used in the vaccine formulation was assumed to be the same as the response to baits filled with actual oral rabies vaccine. Since bait handling did not differ significantly between bait types A, B, and C, it can be concluded that dogs would manipulate a bait filled with oral rabies vaccine similar to the way handling was observed in type A and B baits. Bait type D, containing denatonium benzoate, was considered a negative control. However, bait acceptance and handling time of bait type D did mostly not differ from the other bait types. While these results suggest that bait filling cannot be detected through the sachet and coating by dogs, they also underscore the fact that perception of smell and taste are not necessarily identical among species. Denatonium benzoate has been used as an aversive agent for rodents [
43], but since no studies on the use of denatonium benzoate in dogs exist to date, it cannot be ruled out that the concentration used in our studies was too low for dogs to detect bitterness as intended.
A promising result is the fact that, even though dogs were unfamiliar with the baits offered to them, acceptance rate was generally high. However, it should be noted that bait acceptance and handling on the first day differed from subsequent days. On day 1 of the study, not only was bait acceptance lower (
Table 3), but the animals also chewed the baits for a longer period of time (
Figure 6). On the 4th day, a significantly shorter handling time was observed, but this did not differ between bait types. Overall, the sachet was more often discarded during the last days of the study (
Table 8). The fact that baits were manipulated less by dogs on the 3rd and 4th days of the study also explains why less staining was generally observed on these last two test days. In summary, these findings suggest that there is a certain effect of conditioning to newly offered bait. While dogs were hesitant to approach the bait at first, they started to develop more effective methods to consume only the bait mass itself. This is evidenced in the increase in discarding of the sachet, as swallowing allows the dog to consume as much of the bait mass as possible if they are initially unable to separate it from the sachet. While this conditioning effect has led to better acceptance rates and a shorter handling time, it may also hinder effective bait delivery during single-day ORV campaigns. Still, overall bait acceptance was 91.5% (
Table 2), implying that even in populations wholly unfamiliar with oral rabies vaccine baits, sufficient vaccination coverage can be achieved. Pre-baiting with placebo baits prior to the actual campaign may improve bait acceptance overall. However, these improvements may only be marginal and would have to be evaluated for cost-effectiveness. Furthermore, since animals participating in this study were being kept for other study purposes and were therefore accustomed to human interaction and observation, these results could differ under field settings. Nevertheless, study results on free-roaming dogs showed an acceptance rate of 92.8% when offered an identical egg bait, which they had also never encountered before [
44].
Compared to other bait types, type A was swallowed less often (
Table 7). One possible explanation for this might be the bitter taste of the dye itself. Animals were observed trying to separate the sachet from the bait matrix, instead of continuing to chew the bait after perforating it. In many cases, this was not completely possible, which may have discouraged animals from continuing to handle the bait.
Counterintuitively, coloring was also observed in some animals that had not perforated the sachet. Due to the manual filling of sachets, complete sealing of the dye injection site could not be guaranteed in all cases (S. Ortmann, 2021 personal communication, 8 April). Thus, small amounts of the liquid contents were able to leak out when subjected to pressure by dogs taking up the bait, without actual perforation of the sachet occurring.
Patent Blue V appears to be a promising dye for use as an oral marker substance. In the oral cavity, this dye was especially well visible on the tongue; most likely due to the tongue’s rougher surface compared to the oral mucosa. Although this same dye has been used in previous studies to evaluate bait acceptance, the staining in these studies was found to be less pronounced and was of limited use to evaluate vaccine release in the oral cavity [
22,
23,
24]. This difference is most likely associated with the lower concentration of dye used.
Although visibility of the dye from a distance of 5 m was less pronounced than when examined at close range, the use of Patent Blue V at a concentration such as that used in this study offers many other potential applications. One of these addresses the extreme difficulty to estimate vaccination coverage obtained during oral vaccination campaigns. Per definition, the target population of ORV campaigns are animals that cannot be restrained and handled without special effort. Hence, there is no possibility to mark these dogs by methods such as collaring. Remote application of a marking substance is not easy and provides unreliable results [
12,
45]. In contrast, staining of the tongue through use of Patent Blue V is often pronounced enough to be easily discernible from a short distance and may remain so for several hours. During this study, it was noted that dogs tested earliest in the day (09:00 am IDT) and which had been fed type B baits still showed visible signs of oral staining after trials had concluded (02:00 pm IDT). A capture-recapture survey conducted on the same day, after a vaccination campaign using placebo vaccine baits containing marker dye, may thus provide the opportunity to approximate vaccination coverage of a free-roaming dog population obtained with ORV under field conditions.
Furthermore, these baits can be used to obtain data evaluating potential human contacts with orally vaccinated dogs, and thereby, vaccine contact. As all oral rabies vaccines are based on replication-competent human pathogens, human direct and indirect contact with viable vaccine virus should be prevented. Although hundreds of millions of oral rabies vaccine baits targeted at different wildlife species have been distributed, predominantly in Europe and North America [
34,
46,
47,
48], only two serious adverse events in humans have been reported so far [
49,
50]. Nevertheless, when baits are distributed, targeting free-roaming dogs, the chances that humans will come into contact with the vaccine virus is much higher than when baits are distributed in the context of oral vaccination of wildlife against rabies. Not only do free-roaming dogs and humans share the same environment, but there is also often direct contact between them. It has been shown that viable oral rabies vaccine released in the oral cavity of dogs can be re-isolated from saliva collected several hours after the vaccine bait was offered [
51]. Hence, the possibility of vaccine virus transmission through dogs biting or licking humans remains. The persistence of visible staining by Patent Blue V is similar to the persistence of viable vaccine virus in the oral cavity after oral vaccination. Additionally, it can be assumed that the intensity of staining would correlate with the amount of vaccine virus released in the oral cavity. Hence, during a follow-up survey after distribution of bait containing the marker substance, it could be estimated how many people would have had contact with the contents of ORV bait, as the dye also stains clothes and human skin.
None of the dogs in this study showed any adverse reaction after consuming the multiple baits offered to them during the study period of 4 days. In cases where the sachets were swallowed, staff were able to retrieve remnants of egested material from enclosures the next day. This implies that repeat ingestion of the bait itself, were it to occur under field conditions, would most likely not pose a risk to dogs. It should also be noted that in some cases where type A or B baits were consumed, traces of dye were visible in feces the following day. Nevertheless, this was an unexpected finding. Since complete separation of dogs for the entire study period was neither planned nor spontaneously possible, effects of type A versus type B baits cannot be distinguished in this respect.
We were able to discern individual changes in behavioral patterns regarding bait acceptance and handling over the course of our study using the selected study design. However, the fact that dogs gained increasing familiarity with baits could also impact the results. While this has to be taken into consideration when evaluating the efficacy of the ORV bait used in our study in general, it does not change the fact that, when compared directly to each other, the marker substance Patent Blue V has been shown to have stronger dyeing capacities than Green S, without there being any prominent difference in handling of baits filled with Patent Blue V compared to those filled with ORV-medium alone.