Recent Advances in Developing Treatments of Kaposi’s Sarcoma Herpesvirus-Related Diseases
Abstract
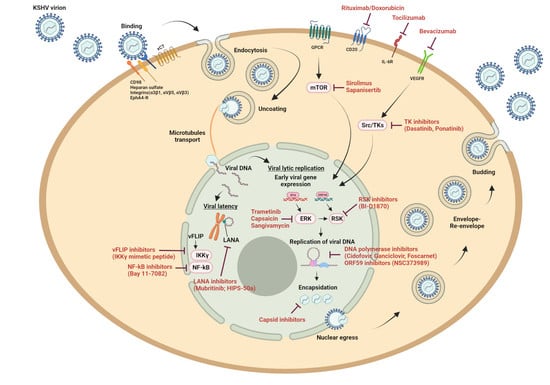
:1. Introduction
2. KSHV DNA Polymerase Inhibitors
3. Antivirals Targeting Other Steps in the Viral Life Cycle
4. Cellular Targets to Inhibit KSHV Replication
4.1. Kinase Inhibitors
4.2. HSP90 and HSP70 Inhibitors
4.3. Other Cellular Targets
5. Monoclonal Antibodies and Immunomodulatory Therapies
6. KSHV Tropism and Models to Study the Virus
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.; Lee, F.; Culpepper, J.; Knowles, D.; Moore, P. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; D’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86, 1276–1280. [Google Scholar] [CrossRef] [Green Version]
- Cesarman, E.; Chang, Y.; Moore, P.; Said, J.W.; Knowles, D.M. Kaposi’s Sarcoma–Associated Herpesvirus-Like DNA Sequences in AIDS-Related Body-Cavity–Based Lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Longnecker, R.; Neipel, F. Introduction to the human γ-herpesviruses. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Eds.; Cambridge Ubiversity Press: Cambridge, UK, 2007. [Google Scholar]
- Uldrick, T.S.; Wang, V.; O’Mahony, D.; Aleman, K.; Wyvill, K.M.; Marshall, V.; Steinberg, S.M.; Pittaluga, S.; Maric, I.; Whitby, D.; et al. An Interleukin-6–Related Systemic Inflammatory Syndrome in Patients Co-Infected with Kaposi Sarcoma–Associated Herpesvirus and HIV but without Multicentric Castleman Disease. Clin. Infect. Dis. 2010, 51, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Matsushima, A.Y.; Strauchen, J.A.; Lee, G.; Scigliano, E.; Hale, E.E.; Weisse, M.T.; Burstein, D.; Kamel, O.; Moore, P.S.; Chang, Y. Posttransplantation Plasmacytic Proliferations Related to Kaposi’s Sarcoma–Associated Herpesvirus. Am. J. Surg. Pathol. 1999, 23, 1393. [Google Scholar] [CrossRef] [PubMed]
- Kapelushnik, J.; Ariad, S.; Benharroch, D.; Landau, D.; Moser, A.; Delsol, G.; Brousset, P. Post renal transplantation human herpesvirus 8-associated lymphoproliferative disorder and Kaposi’s sarcoma. Br. J. Haematol. 2001, 113, 425–428. [Google Scholar] [CrossRef]
- Dupin, N.; Diss, T.L.; Kellam, P.; Tulliez, M.; Du, M.-Q.; Sicard, D.; Weiss, R.A.; Isaacson, P.G.; Boshoff, C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8–positive plasmablastic lymphoma. Blood 2000, 95, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, J.M. Ultrastructure of Kaposi Sarcoma. Ultrastruct. Pathol. 2008, 32, 211–220. [Google Scholar] [CrossRef]
- Antman, K.; Chang, Y. Kaposi’s sarcoma. N. Engl. J. Med. 2000, 342, 1027–1038. [Google Scholar] [CrossRef]
- Kaposi, M. Idiopathisches multiples Pigmentsarkom der Haut. Arch. Dermatol. Syph. 1872, 4, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Plancoulaine, S.; Abel, L.; van Beveren, M.; Trégouët, D.A.; Joubert, M.; Tortevoye, P. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet 2000, 356, 1062–1065. [Google Scholar] [CrossRef]
- Dedicoat, M.; Newton, R.; Alkharsah, K.R.; Sheldon, J.; Szabados, I.; Ndlovu, B. Mother-to-Child Transmission of Human Herpesvirus-8 in South Africa. J. Infect. Dis. 2004, 190, 1068–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebbé, C.; Legendre, C.; Francès, C. Kaposi sarcoma in transplantation. Transplant. Rev. 2008, 22, 252–261. [Google Scholar] [CrossRef]
- Cattani, P.; Capuano, M.; Graffeo, R.; Ricci, R.; Cerimele, F.; Cerimele, D.; Nanni, G.; Fadda, G. Kaposi’s Sarcoma Associated with Previous Human Herpesvirus 8 Infection in Kidney Transplant Recipients. J. Clin. Microbiol. 2001, 39, 506–508. [Google Scholar] [CrossRef] [Green Version]
- Grulich, E.A.; van Leeuwen, M.; Falster, M.; Vajdic, C. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Francès, C.; Marcelin, A.G.; Legendre, C.; Chevret, S.; Dussaix, E.; Lejeune, J.T. The impact of Preexisting or Acquired Kaposi Sarcoma Herpesvirus infection in Kidney Trasplant Recipiens on Morbidity and Survival. Am. J. Transpl. 2009, 9, 2580–2586. [Google Scholar] [CrossRef]
- Wabinga, H.R.; Nambooze, S.; Amulen, P.M.; Okello, C.; Mbus, L.; Parkin, D.M. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int. J. Cancer. 2014, 135, 432–439. [Google Scholar] [CrossRef]
- Friedman-Kien, A.E. Disseminated Kaposi’s sarcoma syndrome in young homosexual men. J. Am. Acad. Dermatol. 1981, 5, 468–471. [Google Scholar] [CrossRef]
- Dupin, N.; De Cervens, V.R.; Gorin, I.; Calvez, V.; Pessis, E.; Grandadam, M.; Rabian, C.; Viard, J.P.; Huraux, J.M.; Escande, J.P. The influence of highly active antiretroviral therapy on AIDS-associated Kaposi’s sarcoma. Br. J. Dermatol. 1999, 140, 875–881. [Google Scholar] [CrossRef]
- Cattelan, A.; Calabro’, M.; Aversa, S.; Zanchetta, M.; Meneghetti, F.; De Rossi, A.; Chieco-Bianchi, L. Regression of AIDS-related Kaposi’s sarcoma following antiretroviral therapy with protease inhibitors: Biological correlates of clinical outcome. Eur. J. Cancer 1999, 35, 1809–1815. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Vajdic, C.; Middleton, M.G.; McDonald, A.M.; Law, M.; Kaldor, J.M.; Grulich, A.E. Continuing declines in some but not all HIV-associated cancers in Australia after widespread use of antiretroviral therapy. AIDS 2009, 23, 2183–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grulich, A.E.; Li, Y.; McDonald, A.M.; Correll, P.K.; Law, M.G.; Kaldor, J.M. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination anti-retroviral therapy. AIDS 2001, 15, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.M.; Inghirami, G.; Ubriaco, A.; Dalla-Favera, R. Molecular Genetic Analysis of Three AIDS-Associated Neoplasms of Uncertain Lineage Demonstrates Their B-Cell Derivation and the Possible Pathogenetic Role of the Epstein-Barr Virus. Blood 1989, 73, 792–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.G.; Zhang, Q.Y.; Lu, Z.B.; Quinto, T.; Rozenvald, I.B.; Liu, L.-T. Extracavitary KSHV-associated large B-Cell lymphoma: A distinct entity or a subtype of primary effusion lymphoma? Study of 9 cases and review of an additional 43 cases. Am. J. Surg. Pathol. 2012, 36, 1129–1140. [Google Scholar] [CrossRef]
- Boulanger, E.; Afonso, P.; Yahiaoui, Y.; Adle-Biassette, H.; Gabarre, J.; Agbalika, F. Human Herpesvirus-8 (HHV-8)-Associated Primary Effusion Lymphoma in two Renal Transplant Recipients Receiving Rapamycin. Arab. Archaeol. Epigr. 2008, 8, 707–710. [Google Scholar] [CrossRef]
- Nador, R.G.; Cesarman, E.; Chadburn, A.; Dawson, D.B.; Ansari, M.Q.; Sald, J.; Knowles, D.M. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 1996, 88, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Jaffe, E.S. HHV-8–positive but EBV-negative primary effusion lymphoma. Blood 2013, 122, 3712. [Google Scholar] [CrossRef] [Green Version]
- Castleman, B.; Towne, V.W. Case Records of the Massachusetts General Hospital: Case No. 40231. N. Engl. J. Med. 1954, 250, 1001–1005. [Google Scholar] [CrossRef]
- Du, M.-Q.; Liu, H.; Diss, T.C.; Ye, H.; Hamoudi, R.A.; Dupin, N. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 2001, 97, 2130–2136. [Google Scholar] [CrossRef]
- Aoki, Y.; Tosato, G.; Fonville, T.W.; Pittaluga, S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood 2001, 97, 2526–2527. [Google Scholar] [CrossRef] [Green Version]
- Oksenhendler, E.; Boulanger, E.; Galicier, L.; Du, M.-Q.; Dupin, N.; Diss, T.C.; Hamoudi, R.; Daniel, M.-T.; Agbalika, F.; Boshoff, C.; et al. High incidence of Kaposi sarcoma–associated herpesvirus–related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002, 99, 2331–2336. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Uldrick, T.S.; Hu, D.; Yarchoan, R. Clinical Manifestations of Kaposi Sarcoma Herpesvirus Lytic Activation: Multicentric Castleman Disease (KSHV–MCD) and the KSHV Inflammatory Cytokine Syndrome. Front. Microbiol. 2012, 3, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedes, D.H.; Ganem, D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Investig. 1997, 99, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Medveczky, M.M.; Horvath, E.; Lund, T.; Medveczky, P.G. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. AIDS 1997, 11, 1327–1332. [Google Scholar] [CrossRef]
- Neyts, J.; De Clercq, E. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob. Agents Chemother. 1997, 41, 2754–2756. [Google Scholar] [CrossRef] [Green Version]
- Sergerie, Y.; Boivin, G. Evaluation of Susceptibility of Human Herpesvirus 8 to Antiviral Drugs by Quantitative Real-Time PCR. J. Clin. Microbiol. 2003, 41, 3897–3900. [Google Scholar] [CrossRef] [Green Version]
- Coen, N.; Singh, U.; Vuyyuru, V.; Oord, J.J.V.D.; Balzarini, J.; Duraffour, S.; Snoeck, R.; Cheng, Y.C.; Chu, C.K.; Andrei, G. Activity and Mechanism of Action of HDVD, a Novel Pyrimidine Nucleoside Derivative with High Levels of Selectivity and Potency against Gammaherpesviruses. J. Virol. 2013, 87, 3839–3851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, N.; Duraffour, S.; Topalis, D.; Snoeck, R.; Andrei, G. Spectrum of Activity and Mechanisms of Resistance of Various Nucleoside Derivatives against Gammaherpesviruses. Antimicrob. Agents Chemother. 2014, 58, 7312–7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luppi, M.; Trovato, R.; Barozzi, P.; Vallisa, D.; Rossi, G.; Re, A.; Ravazzini, L.; Potenza, L.; Riva, G.; Morselli, M.; et al. Treatment of herpesvirus associated primary effusion lymphoma with intracavity cidofovir. Leukemia 2005, 19, 473–476. [Google Scholar] [CrossRef]
- Casper, C.; Krantz, E.M.; Corey, L.; Kuntz, S.R.; Wang, J.; Selke, S.; Hamilton, S.; Huang, M.L.; Wald, A. Valganciclovir for suppression of human herpesvirus-8 replication: A randomized, Double-blind, Placebo-Controlled, Crossover trial. J. Infect. Dis. 2008, 198, 23–30. [Google Scholar] [CrossRef]
- Cattamanchi, A.; Saracino, M.; Selke, S.; Huang, M.-L.; Magaret, A.; Celum, C.; Corey, L.; Wald, A.; Casper, C. Treatment with valacyclovir, famciclovir, or antiretrovirals reduces human herpesvirus-8 replication in HIV-1 seropositive men. J. Med. Virol. 2011, 83, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzi, R.; Parisi, S.G.; Sarmati, L.; Uccella, I.; Nicastri, E.; Carolo, G.; Gatti, F.; Concia, E.; Andreoni, M. Efficacy of cidofovir on human herpesvirus 8 viraemia and Kaposi’s sarcoma progression in two patients with AIDS. AIDS 2001, 15, 2061–2062. [Google Scholar] [CrossRef]
- Simonart, T.; Noel, J.C.; De Dobbeleer, G.; Parent, D.; Van Vooren, J.P.; De Clercq, E. Treatment of Classical Kaposi’s Sarcoma With Intralesional Injections of Cidofovir: Report of a Case. 1998. J. Med. Virol. 1998, 55, 215–218. [Google Scholar] [CrossRef]
- Little, R.F.; Merced-Galindez, F.; Staskus, K.; Whitby, D.; Aoki, Y.; Humphrey, R.; Pluda, J.M.; Marshall, V.; Walters, M.; Welles, L.; et al. A Pilot Study of Cidofovir in Patients with Kaposi Sarcoma. J. Infect. Dis. 2003, 187, 149–153. [Google Scholar] [CrossRef]
- Krown, S.E.; Dittmer, D.P.; Cesarman, E. Pilot Study of Oral Valganciclovir Therapy in Patients With Classic Kaposi Sarcoma. J. Infect. Dis. 2011, 203, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Plachouri, K.; Oikonomou, C.; Sarantopoulos, A.; Koumoundourou, D.; Georgiou, S.; Spiliopoulos, T. Successful treatment and durable remission of human herpesvirus-8-induced Kaposi sarcoma and multicentric Castleman’s disease under valganciclovir in an HIV -negative patient. Dermatol. Ther. 2020, 33, e13419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Burnette, A.; Dorjsuren, D.; Roberts, P.E.; Huleihel, M.; Shoemaker, R.H.; Marquez, V.E.; Agbaria, R.; Sei, S. Potent Antiviral Activity of North-Methanocarbathymidine against Kaposi’s Sarcoma-Associated Herpesvirus. Antimicrob. Agents Chemother. 2005, 49, 4980–4988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, N.; Duraffour, S.; Snoeck, R.; Andrei, G. KSHV Targeted Therapy: An Update on Inhibitors of Viral Lytic Replication. Viruses 2014, 6, 4731–4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prichard, M.N.; Williams, J.D.; Komazin-Meredith, G.; Khan, A.R.; Price, N.B.; Jefferson, G.M.; Harden, E.A.; Hartline, C.B.; Peet, N.P.; Bowlin, T.L. Synthesis and Antiviral Activities of Methylenecyclopropane Analogs with 6-Alkoxy and 6-Alkylthio Substitutions That Exhibit Broad-Spectrum Antiviral Activity against Human Herpesviruses. Antimicrob. Agents Chemother. 2013, 57, 3518–3527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauclair, G.; Naimo, E.; Dubich, T.; Rückert, J.; Koch, S.; Dhingra, A.; Wirth, D.; Schulz, T.F. Targeting Kaposi’s Sarcoma-Associated Herpesvirus ORF21 Tyrosine Kinase and Viral Lytic Reactivation by Tyrosine Kinase Inhibitors Approved for Clinical Use. J. Virol. 2020, 94, e01791-19. [Google Scholar] [CrossRef]
- Gustafson, E.A.; Schinazi, R.F.; Fingeroth, J.D. Human Herpesvirus 8 Open Reading Frame 21 Is a Thymidine and Thymidylate Kinase of Narrow Substrate Specificity That Efficiently Phosphorylates Zidovudine but Not Ganciclovir. J. Virol. 2000, 74, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Pastore, R.D.; Chadburn, A.; Kripas, C.; Schattner, E.J. Novel association of haemophagocytic syndrome with Kaposi’s sarcoma-associated herpesvirus-related primary effusion lymphoma. Br. J. Haematol. 2000, 111, 1112–1115. [Google Scholar]
- Sbenghe, M.M.; Besa, E.; Mahipal, A.; Florea, A.D.; Bray, P.; Caro, J. HHV-8–Associated Multicentric Castleman’s Disease in HIV-Negative Patient: A Novel Therapy for an Orphan Disease. Oncologist. 2012, 17, 145–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozbalak, M.; Tokatli, I.; Özdemirli, M.; Tecimer, T.; Ar, M.C.; Ornek, S.; Koroglu, A.; Laleli, Y.; Ferhanoglu, B. Is valganciclovir really effective in primary effusion lymphoma: Case report of an HIV(−) EBV(−) HHV8(+) patient. Eur. J. Haematol. 2013, 91, 467–469. [Google Scholar] [CrossRef]
- Kantarci, F.E.N.; Eren, R.; Gündoğan, C.; Huq, G.E.; Doğu, M.H.; Suyanı, E.; Gündoğdu, C. A HHV-8 positive, HIV negative multicentric Castleman disease treated with R-CEOP chemotherapy and valganciclovir combination. J. Infect. Chemother. 2016, 22, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Hawkes, E.; Chionh, F.; Chong, G.; Fracp, C.M.M.B. Durable remission of both multicentric Castleman’s disease and Kaposi’s sarcoma with valganciclovir, rituximab and liposomal doxorubicin in an HHV-8-positive, HIV-negative patient. J. Clin. Pharm. Ther. 2016, 42, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Uldrick, T.S.; Polizzotto, M.; Aleman, K.; O’Mahony, D.; Wyvill, K.M.; Wang, V.; Marshall, V.; Pittaluga, S.; Steinberg, S.M.; Tosato, G.; et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: A pilot study of virus-activated cytotoxic therapy. Blood 2011, 117, 6977–6986. [Google Scholar] [CrossRef] [Green Version]
- Dittmer, D.; Damania, B. Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)—an update. Curr. Opin. Virol. 2013, 3, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Wang, H.; Herndier, B.; Ganem, D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 1996, 93, 6641–6646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballestas, M.E.; Chatis, P.A.; Kaye, K.M. Efficient Persistence of Extrachromosomal KSHV DNA Mediated by Latency-Associated Nuclear Antigen. Science 1999, 284, 641–644. [Google Scholar] [CrossRef]
- Dittmer, D.; Lagunoff, M.; Renne, R.; Staskus, K.; Haase, A.; Ganem, D. A Cluster of Latently Expressed Genes in Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 1998, 72, 8309–8315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, K.; Kuppers, D.A.; Verma, S.C.; Sharma, N.; Murakami, M.; Robertson, E.S.; Cheung, C.Y.; Poon, L.L.M.; Ng, I.H.Y.; Luk, W.; et al. Induction of Kaposi’s Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen by the Lytic Transactivator RTA: A Novel Mechanism for Establishment of Latency. J. Virol. 2005, 79, 7819–7826. [Google Scholar] [CrossRef] [Green Version]
- Lan, K.; Kuppers, D.A.; Verma, S.C.; Robertson, E.S. Kaposi’s Sarcoma-Associated Herpesvirus-Encoded Latency-Associated Nuclear Antigen Inhibits Lytic Replication by Targeting Rta: A Potential Mechanism for Virus-Mediated Control of Latency. J. Virol. 2004, 78, 6585–6594. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lu, S.; Zhang, Z.; Gonzalez, C.M.; Damania, B.; Cullen, B.R. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5570–5575. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Veettil, M.V.; Chandran, B. Kaposi’s Sarcoma Associated Herpesvirus Entry into Target Cells. Front. Microbiol. 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupin, N.; Fisher, C.; Kellam, P.; Ariad, S.; Tulliez, M.; Franck, N. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 1999, 96, 4546–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfrey, A.; Anderson, J.; Papanastasiou, A.; Takeuchi, Y.; Boshoff, C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood 2005, 105, 2510–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curreli, F.; Friedman-Kien, A.E.; Flore, O. Glycyrrhizic acid alters Kaposi sarcoma–associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J. Clin. Invest. 2005, 115, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Sin, S.-H.; Wen, K.W.; Damania, B.; Dittmer, D.P. Hsp90 Inhibitors Are Efficacious against Kaposi Sarcoma by Enhancing the Degradation of the Essential Viral Gene LANA, of the Viral Co-Receptor EphA2 as well as Other Client Proteins. PLoS Pathog. 2012, 8, e1003048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tso, F.Y.; West, J.T.; Wood, C. Reduction of Kaposi’s Sarcoma-Associated Herpesvirus Latency Using CRISPR-Cas9 To Edit the Latency-Associated Nuclear Antigen Gene. J. Virol. 2019, 93, e02183-18. [Google Scholar] [CrossRef] [Green Version]
- Haddad, C.O.; Kalt, I.; Shovman, Y.; Xia, L.; Schlesinger, Y.; Sarid, R.; Parnas, O. Targeting the Kaposi’s sarcoma-associated herpesvirus genome with the CRISPR-Cas9 platform in latently infected cells. Virol. J. 2021, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ballestas, M.; Kaye, K.M. Kaposi’s Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen 1 Mediates Episome Persistence through cis -Acting Terminal Repeat (TR) Sequence and Specifically Binds TR DNA. J. Virol. 2001, 75, 3250–3258. [Google Scholar] [CrossRef] [Green Version]
- Barbera, J.A.; Ballestas, M.E.; Kaye, K.M. The Kaposi’s Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen 1 N Terminus Is Essential for Chromosome Association, DNA Replication, and Episome Persistence. J. Virol. 2004, 78, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Hellert, J.; Weidner-Glunde, M.; Krausze, J.; Lünsdorf, H.; Ritter, C.; Schulz, T.F.; Lührs, T. The 3D structure of Kaposi sarcoma herpesvirus LANA C-terminal domain bound to DNA. Proc. Natl. Acad. Sci. USA 2015, 112, 6694–6699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidner-Glunde, M.; Mariggiò, G.; Schulz, T.F. Kaposi’s Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen: Replicating and Shielding Viral DNA during Viral Persistence. J. Virol. 2017, 91, e01083-16. [Google Scholar] [CrossRef] [Green Version]
- Domsic, J.F.; Chen, H.-S.; Lu, F.; Marmorstein, R.; Lieberman, P.M. Molecular Basis for Oligomeric-DNA Binding and Episome Maintenance by KSHV LANA. PLoS Pathog. 2013, 9, e1003672. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, P.E.; Jakob, V.; Oberhausen, K.; Stein, S.C.; Cucarro, I.S.; Schulz, T.F.; Empting, M. Fragment-Based Discovery of a Qualified Hit Targeting the Latency-Associated Nuclear Antigen of the Oncogenic Kaposi’s Sarcoma-Associated Herpesvirus/Human Herpesvirus 8. J. Med. Chem. 2019, 62, 3924–3939. [Google Scholar] [CrossRef]
- Kirsch, P.; Stein, S.C.; Berwanger, A.; Rinkes, J.; Jakob, V.; Schulz, T.F.; Empting, M. Hit-to-lead optimization of a latency-associated nuclear antigen inhibitor against Kaposi’s sarcoma-associated herpesvirus infections. Eur. J. Med. Chem. 2020, 202, 112525. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P.; Jakob, V.; Elgaher, W.A.M.; Walt, C.; Oberhausen, K.; Schulz, T.; Empting, M. Discovery of Novel Latency-Associated Nuclear Antigen Inhibitors as Antiviral Agents Against Kaposi’s Sarcoma-Associated Herpesvirus. ACS Chem. Biol. 2020, 15, 388–395. [Google Scholar] [CrossRef]
- Calderon, A.; Soldan, S.S.; De Leo, A.; Deng, Z.; Frase, D.M.; Anderson, E.M.; Zhang, Y.; Vladimirova, O.; Lu, F.; Leung, J.C.; et al. Identification of Mubritinib (TAK 165) as an inhibitor of KSHV driven primary effusion lymphoma via disruption of mitochondrial OXPHOS metabolism. Oncotarget 2020, 11, 4224–4242. [Google Scholar] [CrossRef]
- Keller, S.A.; Schattner, E.J.; Cesarman, E. Inhibition of NF-B Induces Apoptosis of KSHV-Infected Primary Effusion Lymphoma Cells. 2000. Available online: https://ashpublications.org/blood/article-pdf/96/7/2537/1668374/h8190002537.pdf (accessed on 30 August 2021).
- Chugh, P.; Matta, H.; Schamus, S.; Zachariah, S.; Kumar, A.; Richardson, J.A.; Smith, A.L.; Chaudhary, P.M. Constitutive NF- B activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc. Natl. Acad. Sci. USA 2005, 102, 12885–12890. [Google Scholar] [CrossRef] [Green Version]
- Guasparri, I.; Keller, S.A.; Cesarman, E. KSHV vFLIP Is Essential for the Survival of Infected Lymphoma Cells. J. Exp. Med. 2004, 199, 993–1003. [Google Scholar] [CrossRef]
- Keller, S.A.; Hernandez-Hopkins, D.; Vider, J.; Ponomarev, V.; Hyjek, E.; Schattner, E.J.; Cesarman, E. NF-κB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood 2006, 107, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Baloon, G.; Chen, K.; Perez, R.; Tam, W.; Cesarman, E. Kaposi Sarcoma Herpesvirus (KSHV) VFLIP Oncoprotein Induces B Cell Transdifferentiation and Tumorigenesis in Mice. J. Clin. Investig. 2011, 121, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Sadek, J.; Wuo, M.G.; Rooklin, D.; Hauenstein, A.; Hong, S.H.; Gautam, A. Modulation of virus-induced NF-κB signaling by NEMO coiled coil mimics. Nat Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Field, N.; Low, W.; Daniels, M.; Howell, S.; Daviet, L.; Boshoff, C.; Collins, M. KSHV vFLIP binds to IKK-γ to activate IKK. J. Cell Sci. 2003, 116, 3721–3728. [Google Scholar] [CrossRef] [Green Version]
- Bagnéris, C.; Ageichik, A.V.; Cronin, N.; Wallace, B.; Collins, M.; Boshoff, C.; Waksman, G.; Barrett, T. Crystal Structure of a VFlip-IKKγ Complex: Insights into Viral Activation of the IKK Signalosome. Mol. Cell 2008, 30, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.C.; Chan, A.W.E.; Davis, C.A.; Whitelock, N.; Hotiana, H.A.; Baratchian, M.; Bagnéris, C.; Selwood, D.L.; Collins, M.K.; Barrett, T.E. IKKγ-Mimetic Peptides Block the Resistance to Apoptosis Associated with Kaposi’s Sarcoma-Associated Herpesvirus Infection. J. Virol. 2017, 91, e01170-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.X.; Cusano, T.; Yuan, Y. Identification of the Immediate-Early Transcripts of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 1999, 73, 5556–5567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purushothaman, P.; Uppal, T.; Verma, S.C. Molecular Biology of KSHV Lytic Reactivation. Viruses 2015, 7, 116–153. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Lin, S.-F.; Gradoville, L.; Yuan, Y.; Zhu, F.; Miller, G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 1998, 95, 10866–10871. [Google Scholar] [CrossRef] [Green Version]
- Long, W.; Zhao, G.; Wu, Y.; Liu, Y. Gallic acid inhibits Kaposi’s Sarcoma-associated herpesvirus lytic reactivation by suppressing RTA transcriptional activities. Food Sci. Nutr. 2020, 9, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Dorjsuren, D.; Burnette, A.; Gray, G.N.; Chen, X.; Zhu, W.; Roberts, P.E.; Currens, M.J.; Shoemaker, R.H.; Ricciardi, R.P.; Sei, S. Chemical library screen for novel inhibitors of Kaposi’s sarcoma-associated herpesvirus processive DNA synthesis. Antivir. Res. 2006, 69, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Bryant, K.F.; Gregory, S.M.; Angelova, M.; Dreyfus, D.H.; Zhao, X.Z.; Coen, D.M.; Burke, T.R.; Knipe, D.M. HIV Integrase Inhibitors Block Replication of Alpha-, Beta-, and Gammaherpesviruses. mBio 2014, 5, e01318-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.T.; Zhao, H.; Masaoka, T.; Varnado, B.; Castro, E.M.C.; Marshall, V.A.; Kouhestani, K.; Lynn, A.Y.; Aron, K.E.; Xia, A.; et al. Sensitivity of the C-Terminal Nuclease Domain of Kaposi’s Sarcoma-Associated Herpesvirus ORF29 to Two Classes of Active-Site Ligands. Antimicrob. Agents Chemother. 2018, 62, e00233-18. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, W.W.; Brown, J.C. Inhibition of Herpes Simplex Virus Replication by WAY-150138: Assembly of Capsids Depleted of the Portal and Terminase Proteins Involved in DNA Encapsidation. J. Virol. 2002, 76, 10084–10088. [Google Scholar] [CrossRef] [Green Version]
- Visalli, R.J.; Fairhurst, J.; Srinivas, S.; Hu, W.; Feld, B.; DiGrandi, M.; Curran, K.; Ross, A.; Bloom, J.D.; van Zeijl, M.; et al. Identification of Small Molecule Compounds That Selectively Inhibit Varicella-Zoster Virus Replication. J. Virol. 2003, 77, 2349–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visalli, R.J.; Van Zeijl, M. DNA encapsidation as a target for anti-herpesvirus drug therapy. Antivir. Res. 2003, 59, 73–87. [Google Scholar] [CrossRef]
- Kornfeind, E.M.; Visalli, R.J. Human herpesvirus portal proteins: Structure, function, and antiviral prospects. Rev. Med. Virol. 2018, 28, e1972. [Google Scholar] [CrossRef]
- Goldner, T.; Hewlett, G.; Ettischer, N.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. The Novel Anticytomegalovirus Compound AIC246 (Letermovir) Inhibits Human Cytomegalovirus Replication through a Specific Antiviral Mechanism That Involves the Viral Terminase. J. Virol. 2011, 85, 10884–10893. [Google Scholar] [CrossRef] [Green Version]
- Unal, A.; Pray, T.R.; Lagunoff, M.; Pennington, M.W.; Ganem, D.; Craik, C.S. The protease and the assembly protein of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8). J. Virol. 1997, 71, 7030–7038. [Google Scholar] [CrossRef] [Green Version]
- Shahian, T.; Lee, G.M.; Lazic, A.; Arnold, L.A.; Velusamy, P.; Roels, C.M.; Guy, R.K.; Craik, C.S. Inhibition of a viral enzyme by a small-molecule dimer disruptor. Nat. Chem. Biol. 2009, 5, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Brulois, K.; Wong, L.; Jung, J.U. Modulation of Immune System by Kaposi’s Sarcoma-Associated Herpesvirus: Lessons from Viral Evasion Strategies. Front. Microbiol. 2012, 3, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acker, T.M.; Gable, J.E.; Bohn, M.F.; Jaishankar, P.; Thompson, M.C.; Fraser, J.S.; Renslo, A.R.; Craik, C.S. Allosteric Inhibitors, Crystallography, and Comparative Analysis Reveal Network of Coordinated Movement across Human Herpesvirus Proteases. J. Am. Chem. Soc. 2017, 139, 11650–11653. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Carlsson, J.; Ikoma, M.; Gachelet, E.; Gray, M.; Geballe, A.P.; Corey, L.; Casper, C.; Lagunoff, M.; Vieira, J. The HIV Protease Inhibitor Nelfinavir Inhibits Kaposi’s Sarcoma-Associated Herpesvirus Replication In Vitro. Antimicrob. Agents Chemother. 2011, 55, 2696–2703. [Google Scholar] [CrossRef] [Green Version]
- Gantt, S.; Cattamanchi, A.; Krantz, E.; Magaret, A.; Selke, S.; Kuntz, S.R.; Huang, M.-L.; Corey, L.; Wald, A.; Casper, C. Reduced human herpesvirus-8 oropharyngeal shedding associated with protease inhibitor-based antiretroviral therapy. J. Clin. Virol. 2014, 60, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Berger, E.A. An Immunotoxin Targeting the GH Glycoprotein of KSHV for Selective Killing of Cells in the Lytic Phase of Infection. Antivir. Res. 2011, 90, 143–1450. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, D.; Chandran, B.; Berger, E.A. Selective Killing of Kaposi’s Sarcoma-Associated Herpesvirus Lytically Infected Cells with a Recombinant Immunotoxin Targeting the Viral GpK8.1A Envelope Glycoprotein. MAbs 2012, 4, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Heston, L.; Grogan, E.; Gradoville, L.; Rigsby, M.; Sun, R.; Shedd, D.; Kushnaryov, V.M.; Grossberg, S.; Chang, Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 1997, 71, 314–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ueda, K.; Sakakibara, S.; Okuno, T.; Parravicini, C.; Corbellino, M.; Yamanishi, K. Activation of latent Kaposi’s sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc. Natl. Acad. Sci. USA 2001, 98, 4119–4124. [Google Scholar] [CrossRef] [Green Version]
- Whitby, D.; Marshall, V.A.; Bagni, R.K.; Miley, W.J.; McCloud, T.G.; Hines-Boykin, R.; Goedert, J.J.; Conde, B.A.; Nagashima, K.; Mikovits, J.; et al. Reactivation of Kaposi’s sarcoma-associated herpesvirus by natural products from Kaposi’s sarcoma endemic regions. Int. J. Cancer 2006, 120, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; He, M.; Zhou, F.; Ye, F.; Gao, S.-J. Activation of Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) by Inhibitors of Class III Histone Deacetylases: Identification of Sirtuin 1 as a Regulator of the KSHV Life Cycle. J. Virol. 2014, 88, 6355–6367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Qin, Z.; Riker, A.I.; Xi, Y. CRISPR/Cas9 ablating viral microRNA promotes lytic reactivation of Kaposi’s sarcoma-associated herpesvirus. Biochem. Biophys. Res. Commun. 2020, 533, 1400–1405. [Google Scholar] [CrossRef]
- Iida, S.; Mine, S.; Ueda, K.; Suzuki, T.; Hasegawa, H.; Katano, H. Suberoyl Bis-Hydroxamic Acid Reactivates Kaposi’s Sarcoma-Associated Herpesvirus through Histone Acetylation and Induces Apoptosis in Lymphoma Cells. J. Virol. 2021, 95, e01785-20. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, E.; Anderton, M.; Blumenthal, M.; Schäfer, G. Cellular Receptors Involved in KSHV Infection. Viruses 2021, 13, 118. [Google Scholar] [CrossRef]
- Koon, H.B.; Bubley, G.J.; Pantanowitz, L.; Masiello, D.; Smith, B.; Crosby, K.; Proper, J.; Weeden, W.; Miller, T.E.; Chatis, P.; et al. Imatinib-Induced Regression of AIDS-Related Kaposi’s Sarcoma. J. Clin. Oncol. 2005, 23, 982–989. [Google Scholar] [CrossRef]
- Koon, H.B.; Krown, S.E.; Lee, J.Y.; Honda, K.; Rapisuwon, S.; Wang, Z.; Aboulafia, D.; Reid, E.G.; Rudek, M.A.; Dezube, B.J.; et al. Phase II Trial of Imatinib in AIDS-Associated Kaposi’s Sarcoma: AIDS Malignancy Consortium Protocol 042. J. Clin. Oncol. 2014, 32, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Ardavanis, A.; Doufexis, D.; Kountourakis, P.; Rigatos, G. A Kaposi’s Sarcoma Complete Clinical Response after Sorafenib Administration. Ann. Oncol. 2008, 19, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Uldrick, T.S.; Gonçalves, P.H.; Wyvill, K.M.; Peer, C.J.; Bernstein, W.; Aleman, K.; Polizzotto, M.; Venzon, D.; Steinberg, S.M.; Marshall, V.; et al. A Phase Ib Study of Sorafenib (BAY 43-9006) in Patients with Kaposi Sarcoma. Oncology 2017, 22, 505. [Google Scholar] [CrossRef] [Green Version]
- Gill, M.B.; Turner, R.; Stevenson, P.G.; Way, M. KSHV-TK is a tyrosine kinase that disrupts focal adhesions and induces Rho-mediated cell contraction. EMBO J. 2014, 34, 448–465. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.P.; Stuhlmiller, T.J.; Giffin, L.C.; Lin, C.; Bigi, R.; Zhao, J.; Zhang, W.; Cruz, A.G.B.; Park, S.I.; Earp, H.S.; et al. Kinome profiling of non-Hodgkin lymphoma identifies Tyro3 as a therapeutic target in primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 2019, 116, 16541–16550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodhi, A.; Chaisuparat, R.; Hu, J.; Ramsdell, A.K.; Manning, B.D.; Sausville, E.A.; Sawai, E.T.; Molinolo, A.; Gutkind, J.S.; Montaner, S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 2006, 10, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Nichols, L.A.; Adang, L.A.; Kedes, D.H. Rapamycin Blocks Production of KSHV/HHV8: Insights into the Anti-Tumor Activity of an Immunosuppressant Drug. PLoS ONE 2011, 6, e14535. [Google Scholar] [CrossRef] [PubMed]
- Sin, S.-H.; Roy, D.; Wang, L.; Staudt, M.R.; Fakhari, F.D.; Patel, D.D.; Henry, D.; Harrington, W.J.; Damania, B.A.; Dittmer, D.P. Rapamycin Is Efficacious against Primary Effusion Lymphoma (PEL) Cell Lines in Vivo by Inhibiting Autocrine Signaling. Blood 2007, 109, 2165–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, A.P.; Bhende, P.M.; Sin, S.H.; Roy, D.; Dittmer, D.P.; Damania, B. Dual Inhibition of PI3K and MTOR Inhibits Autocrine and Paracrine Proliferative Loops in PI3K/Akt/MTOR-Addicted Lymphomas. Blood 2010, 115, 4455–4463. [Google Scholar] [CrossRef]
- Caro-Vegas, C.; Bailey, A.; Bigi, R.; Damania, B.; Dittmer, D.P. Targeting MTOR with MLN0128 Overcomes Rapamycin and Chemoresistant Primary Effusion Lymphoma. MBio 2019, 10, e02871-18. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Xie, J.; Ye, F.; Gao, S.-J. Modulation of Kaposi’s Sarcoma-Associated Herpesvirus Infection and Replication by MEK/ERK, JNK, and p38 Multiple Mitogen-Activated Protein Kinase Pathways during Primary Infection. J. Virol. 2006, 80, 5371–5382. [Google Scholar] [CrossRef] [Green Version]
- Wakao, K.; Watanabe, T.; Takadama, T.; Ui, S.; Shigemi, Z.; Kagawa, H.; Higashi, C.; Ohga, R.; Taira, T.; Fujimuro, M. Sangivamycin induces apoptosis by suppressing Erk signaling in primary effusion lymphoma cells. Biochem. Biophys. Res. Commun. 2014, 444, 135–140. [Google Scholar] [CrossRef]
- Moriguchi, M.; Watanabe, T.; Kadota, A.; Fujimuro, M. Capsaicin Induces Apoptosis in KSHV-Positive Primary Effusion Lymphoma by Suppressing ERK and p38 MAPK Signaling and IL-6 Expression. Front. Oncol. 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, L.; Xiao, Y.; Yao, X.; Long, X.; Zhu, F.; Kuang, E. Development of an ORF45-Derived Peptide To Inhibit the Sustained RSK Activation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2019, 93, e02154-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Trillo-Tinoco, J.; Cao, Y.; Bonstaff, K.; Doyle, L.; del Valle, L.; Whitby, D.; Qin, Z. Targeting HGF/c-MET Induces Cell Cycle Arrest, DNA Damage, and Apoptosis for Primary Effusion Lymphoma. Blood J. Am. Soc. Hematol. 2015, 126, 2821–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayar, U.; Lu, P.; Vider, J.; Cerchietti, L.; Chiosis, G.; Wang, L.; Blasberg, R.; Cesarman, E. Hsp90 is a viable therapeutic target in the treatment of KSHV-associated primary effusion lymphoma. Infect. Agents Cancer 2010, 5, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.W.; Damania, B. Kaposi sarcoma-associated herpesvirus (KSHV): Molecular biology and oncogenesis. Cancer Lett. 2010, 289, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Defee, M.; Isaacs, J.S.; Parsons, C. Extracellular Hsp90 serves as a co-factor for MAPK activation and latent viral gene expression during de novo infection by KSHV. Virology 2010, 403, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Higashi, C.; Saji, C.; Yamada, K.; Kagawa, H.; Ohga, R.; Taira, T.; Fujimuro, M. The Effects of Heat Shock Protein 90 Inhibitors on Apoptosis and Viral Replication in Primary Effusion Lymphoma Cells. Biol. Pharm. Bull. 2012, 35, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, R.; Matta, H.; Chaudhary, P.M. A Purine Scaffold HSP90 Inhibitor BIIB021 Has Selective Activity against KSHV-Associated Primary Effusion Lymphoma and Blocks vFLIP K13-Induced NF-κB. Clin. Cancer Res. 2013, 19, 5016–5026. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.-F.; Kim, Y.-S.; Xiang, S.; Abdullaev, Z.; Torrey, T.A.; Janz, S.; Kovalchuk, A.L.; Sun, J.; Chen, D.; Cho, W.C.; et al. Characterization of ARF-BP1/HUWE1 Interactions with CTCF, MYC, ARF and p53 in MYC-Driven B Cell Neoplasms. Int. J. Mol. Sci. 2012, 13, 6204–6219. [Google Scholar] [CrossRef] [Green Version]
- Baquero-Pérez, B.; Whitehouse, A. Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments. PLoS Pathog. 2015, 11, e1005274. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.; Wood, J.J.; Jackson, B.; Baquero-Perez, B.; Whitehouse, A. NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target. PLoS Pathog. 2015, 11, e1004771. [Google Scholar] [CrossRef] [Green Version]
- Matta, H.; Chaudhary, P.M. The proteasome inhibitor bortezomib (PS-341) inhibits growth and induces apoptosis in primary effusion lymphoma cells. Cancer Biol. Ther. 2005, 4, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Granato, M.; Romeo, M.A.; Tiano, M.S.; Santarelli, R.; Gonnella, R.; Montani, M.S.G.; Faggioni, A.; Cirone, M. Bortezomib promotes KHSV and EBV lytic cycle by activating JNK and autophagy. Sci. Rep. 2017, 7, 13052. [Google Scholar] [CrossRef] [Green Version]
- Sarosiek, K.; Cavallin, L.E.; Bhatt, S.; Toomey, N.L.; Natkunam, Y.; Blasini, W.; Gentles, A.J.; Ramos, J.C.; Mesri, E.A.; Lossos, I.S. Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 2010, 107, 13069–13074. [Google Scholar] [CrossRef] [Green Version]
- Reid, E.G.; Suazo, A.; Lensing, S.Y.; Dittmer, D.P.; Ambinder, R.F.; Maldarelli, F.; Gorelick, R.J.; Aboulafia, D.M.; Mitsuyasu, R.; Dickson, M.A.; et al. Pilot Trial AMC-063: Safety and Efficacy of Bortezomib in AIDS-associated Kaposi Sarcoma. Clin. Cancer Res. 2019, 26, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, H.; Chen, X. Pemetrexed Inhibits Kaposi’s Sarcoma-Associated Herpesvirus Replication through Blocking DTMP Synthesis. Antivir. Res. 2020, 180, 104825. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.; González-Molleda, L.; Wang, Y.; Xu, J.; Yuan, Y. Antiviral Activity of (+)-Rutamarin against Kaposi’s Sarcoma-Associated Herpesvirus by Inhibition of the Catalytic Activity of Human Topoisomerase II. Antimicrob. Agents Chemother. 2013, 58, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.A.; Rinderknecht, A.S.; Zoeteweij, J.P.; Aoki, Y.; Read-Connole, E.L.; Tosato, G.; Blauvelt, A.; Yarchoan, R. Hypoxia Induces Lytic Replication of Kaposi Sarcoma–Associated Herpesvirus. Blood 2001, 97, 3244–3250. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Davis, D.A.; Wang, V.; Widmer, I.; Yarchoan, R. Kaposi’s Sarcoma-Associated Herpesvirus (Human Herpesvirus 8) Contains Hypoxia Response Elements: Relevance to Lytic Induction by Hypoxia. J. Virol. 2003, 77, 6761–6768. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Wang, V.; Davis, D.A.; Zheng, Z.-M.; Yarchoan, R. Genetic Organization and Hypoxic Activation of the Kaposi’s Sarcoma-Associated Herpesvirus ORF34-37 Gene Cluster. J. Virol. 2006, 80, 7037–7051. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, P.; Davis, D.A.; Veeranna, R.P.; Carey, R.F.; Viollet, C.; Yarchoan, R. Hypoxia-inducible factor-1 alpha as a therapeutic target for primary effusion lymphoma. PLoS Pathog. 2017, 13, e1006628. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Tan, B.; Vasan, K.; Yuan, H.; Cheng, F.; da Silva, S.R.; Lu, C.; Gao, S.-J. SIRT1 and AMPK pathways are essential for the proliferation and survival of primary effusion lymphoma cells. J. Pathol. 2017, 242, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qiao, J.; Nguyen, D.; Struckhoff, A.P.; Doyle, L.; Bonstaff, K.; Del Valle, L.; Parsons, C.; Toole, B.P.; Renne, R.; et al. Role of heme oxygenase-1 in the pathogenesis and tumorigenicity of Kaposi’s sarcoma-associated herpesvirus. Oncotarget 2016, 7, 10459–10471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uldrick, T.S.; Wyvill, K.M.; Kumar, P.; O’Mahony, D.; Bernstein, W.; Aleman, K.; Polizzotto, M.; Steinberg, S.M.; Pittaluga, S.; Marshall, V.; et al. Phase II Study of Bevacizumab in Patients With HIV-Associated Kaposi’s Sarcoma Receiving Antiretroviral Therapy. J. Clin. Oncol. 2012, 30, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, R.; Uldrick, T.S.; Polizzotto, M.; Wyvill, K.M.; Goncalves, P.; Widell, A.; Lurain, K.; Steinberg, S.M.; Figg, W.D.; Tosato, G.; et al. A Pilot Study of Liposomal Doxorubicin Combined with Bevacizumab followed by Bevacizumab Monotherapy in Patients with Advanced Kaposi Sarcoma. Clin. Cancer Res. 2019, 25, 4238–4247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uldrick, T.S.; Polizzotto, M.; Aleman, K.; Wyvill, K.M.; Marshall, V.; Whitby, D.; Wang, V.; Pittaluga, S.; O’Mahony, D.; Steinberg, S.M.; et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 2014, 124, 3544–3552. [Google Scholar] [CrossRef] [Green Version]
- Marcelin, A.-G.; Aaron, L.; Mateus, C.; Gyan, E.; Gorin, I.; Viard, J.-P.; Calvez, V.; Dupin, N. Rituximab therapy for HIV-associated Castleman disease. Blood 2003, 102, 2786–2788. [Google Scholar] [CrossRef] [Green Version]
- Neuville, S.; Agbalika, F.; Rabian, C.; Brière, J.; Molina, J.-M. Failure of rituximab in human immunodeficiency virus-associated multicentric Castleman disease. Am. J. Hematol. 2005, 79, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Nakazawa, S.; Hanabusa, H. Short-Term Efficacy of the IL6 Receptor Antibody Tocilizumab in Patients with HIV-Associated Multicentric Castleman Disease: Report of Two Cases. J. Hematol. Oncol. 2014, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Aita, T.; Hamaguchi, S.; Shimotani, Y.; Nakamoto, Y. Idiopathic Multicentric Castleman Disease Preceded by Cutaneous Plasmacytosis Successfully Treated by Tocilizumab. BMJ Case Rep. 2020, 13, e236283. [Google Scholar] [CrossRef]
- Ramaswami, R.; Lurain, K.; Peer, C.J.; Serquiña, A.; Wang, V.Y.; Widell, A.; Goncalves, P.; Steinberg, S.M.; Marshall, V.; George, J.; et al. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus–associated multicentric Castleman disease. Blood 2020, 135, 2316–2319. [Google Scholar] [CrossRef]
- Moodad, S.; El Hajj, R.; Hleihel, R.; Hajjar, L.; Tawil, N.; Karam, M.; Hamie, M.; Merhi, R.A.; El Sabban, M.; El Hajj, H. Lenalidomide in Combination with Arsenic Trioxide: An Effective Therapy for Primary Effusion Lymphoma. Cancers 2020, 12, 2483. [Google Scholar] [CrossRef]
- Shrestha, P.; Davis, D.A.; Jaeger, H.K.; Stream, A.; Aisabor, A.I.; Yarchoan, R. Pomalidomide restores immune recognition of primary effusion lymphoma through upregulation of ICAM-1 and B7-2. PLoS Pathog. 2021, 17, e1009091. [Google Scholar] [CrossRef]
- Polizzotto, M.; Uldrick, T.S.; Wyvill, K.M.; Aleman, K.; Peer, C.J.; Bevans, M.; Sereti, I.; Maldarelli, F.; Whitby, D.; Marshall, V.; et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J. Clin. Oncol. 2016, 34, 4125–4131. [Google Scholar] [CrossRef]
- Neil, R.; Halaby, T.; Weverling, G.J.; Dukers, N.H.T.M.; Simpson, G.R.; Coutinho, R.A.; Lange, J.M.A.; Schulz, T.F.; Goudsmit, J. Seroconversion for Human Herpesvirus 8 during HIV Infection Is Highly Predictive of Kaposi’s Sarcoma. AIDS 1998, 12, 2481–2488. [Google Scholar]
- Blasig, C.; Zietz, C.; Haar, B.; Neipel, F.; Esser, S.; Brockmeyer, N.H.; Tschachler, E.; Colombini, S.; Ensoli, B.; Stürzl, M. Monocytes in Kaposi’s Sarcoma Lesions Are Productively Infected by Human Herpesvirus 8. J. Virol. 1997, 71, 7963–7968. [Google Scholar] [CrossRef] [Green Version]
- Boshoff, C.; Schulz, T.F.; Kennedy, M.M.; Graham, A.K.; Fisher, C.; Thomas, A.; McGee, J.O.; Weiss, R.A.; O’Leary, J.J. Kaposi’s Sarcoma-Associated Herpesvirus Infects Endothelial and Spindle Cells. Nat. Med. 1995, 1, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Flore, O.; Rafii, S.; Ely, S.; O’Leary, J.J.; Hyjek, E.M.; Cesarman, E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature 1998, 394, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Diamond, C.; Brodie, S.J.; Krieger, J.N.; Huang, M.-L.; Koelle, D.M.; Diem, K.; Muthui, D.; Corey, L. Human Herpesvirus 8 in the Prostate Glands of Men with Kaposi’s Sarcoma. J. Virol. 1998, 72, 6223–6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, A.V.; Fish, K.N.; Ruhl, R.; Smith, P.P.; Strussenberg, J.G.; Zhu, L.; Chandran, B.; Nelson, J.A. Long-Term Infection and Transformation of Dermal Microvascular Endothelial Cells by Human Herpesvirus 8. J. Virol. 1999, 73, 6892–6902. [Google Scholar] [CrossRef] [Green Version]
- Blackbourn, D.J.; Lennette, E.; Klencke, B.; Moses, A.; Chandran, B.; Weinstein, M.; Glogau, R.G.; Levy, J.A. The Restricted Cellular Host Range of Human Herpesvirus 8. AIDS 2000, 14, 1123–1133. [Google Scholar] [CrossRef]
- Chandran, B.; Hutt-Fletcher, L. Gammaherpesviruses entry and early events during infection. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Bechtel, J.T.; Liang, Y.; Hvidding, J.; Ganem, D. Host Range of Kaposi’s Sarcoma-Associated Herpesvirus in Cultured Cells. J. Virol. 2003, 77, 6474–6481. [Google Scholar] [CrossRef] [Green Version]
- Ojala, P.M.; Schulz, T. Manipulation of endothelial cells by KSHV: Implications for angiogenesis and aberrant vascular differentiation. Semin. Cancer Biol. 2014, 26, 69–77. [Google Scholar] [CrossRef]
- Dubich, T.; Dittrich, A.; Bousset, K.; Geffers, R.; Büsche, G.; Köster, M.; Hauser, H.; Schulz, T.F.; Wirth, D. 3D culture conditions support Kaposi’s sarcoma herpesvirus (KSHV) maintenance and viral spread in endothelial cells. J. Mol. Med. 2021, 99, 425–438. [Google Scholar] [CrossRef]
- Fujiwara, S.; Nakamura, H. Animal Models for Gammaherpesvirus Infections: Recent Development in the Analysis of Virus-Induced Pathogenesis. Pathogens 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Wachtman, L.M.; Pearson, C.B.; Lee, J.-S.; Lee, H.-R.; Lee, S.H.; Vieira, J.; Mansfield, K.G.; Jung, J.U. Non-Human Primate Model of Kaposi’s Sarcoma-Associated Herpesvirus Infection. PLoS Pathog. 2009, 5, e1000606. [Google Scholar] [CrossRef] [Green Version]
- Orzechowska, B.; Powers, M.F.; Sprague, J.; Li, H.; Yen, B.; Searles, R.P.; Axthelm, M.K.; Wong, S.W. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: Animal model for KSHV-associated malignancies. Blood 2008, 112, 4227–4234. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.U.; Choi, J.-K.; Ensser, A.; Biesinger, B. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 1999, 9, 231–239. [Google Scholar] [CrossRef]
- Efstathiou, S.; Ho, Y.M.; Minson, A.C. Cloning and molecular characterization of the murine herpesvirus 68 genome. J. Gen. Virol. 1990, 71, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.; Latreille, P.; Wamsley, P.; Hallsworth, K.; Weck, E.K.; Canto, A.J.D.; Speck, S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997, 71, 5894–5904. [Google Scholar] [CrossRef] [Green Version]
- Louna, K.; Houshaymi, B.; Abdel-Samad, R.; Jaafar, M.; Halloum, I.; Pisano, C.; Neipel, F.; Darwiche, N.; Merhi, R.A. Antitumor Activity of the Synthetic Retinoid ST1926 on Primary Effusion Lymphoma in Vitro and in Vivo Models. Oncol. Rep. 2018, 39, 721–730. [Google Scholar] [CrossRef] [Green Version]
- McHugh, D.; Caduff, N.; Barros, M.H.M.; Rämer, P.C.; Raykova, A.; Murer, A.; Landtwing, V.; Quast, I.; Styles, C.; Spohn, M.; et al. Persistent KSHV Infection Increases EBV-Associated Tumor Formation In Vivo via Enhanced EBV Lytic Gene Expression. Cell Host Microbe 2017, 22, 61–73.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naimo, E.; Zischke, J.; Schulz, T.F. Recent Advances in Developing Treatments of Kaposi’s Sarcoma Herpesvirus-Related Diseases. Viruses 2021, 13, 1797. https://0-doi-org.brum.beds.ac.uk/10.3390/v13091797
Naimo E, Zischke J, Schulz TF. Recent Advances in Developing Treatments of Kaposi’s Sarcoma Herpesvirus-Related Diseases. Viruses. 2021; 13(9):1797. https://0-doi-org.brum.beds.ac.uk/10.3390/v13091797
Chicago/Turabian StyleNaimo, Eleonora, Jasmin Zischke, and Thomas F. Schulz. 2021. "Recent Advances in Developing Treatments of Kaposi’s Sarcoma Herpesvirus-Related Diseases" Viruses 13, no. 9: 1797. https://0-doi-org.brum.beds.ac.uk/10.3390/v13091797