Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations
Abstract
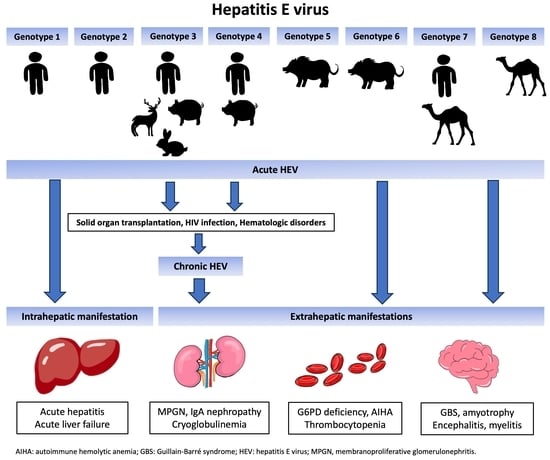
:1. Introduction
2. Epidemiology of Hepatitis E Virus
3. Characteristics and Genetic Diversity of Hepatitis E Virus


4. Hepatitis E Virus Genome
5. Hepatitis E Life Cycle and Host Interaction

6. Diagnosis of HEV Infection
6.1. Laboratory Diagnosis
6.2. Molecular Testing
6.3. Antibody Assays
6.4. Antigen Assays
6.5. Immunochemistry
7. Clinical Manifestations
Hepatic Infection
8. Extrahepatic Complications
8.1. Neurologic Complications
8.2. Hematologic Complications
8.3. Renal Complications
9. Chronic Hepatitis E Virus Infection
9.1. Pathogenesis of Chronic HEV Infection
9.2. Chronic Hepatitis E in Solid-Organ Transplant Recipients
9.3. Liver Transplantation
9.4. Kidney Transplantation and Heart Transplantation
9.5. Hematopoietic Stem Cell Transplantation
10. Chronic HEV Infection in Immunocompromised Patients
10.1. Human Immunodeficiency Virus Infection
10.2. Hematologic Malignancy
10.3. Rheumatological Diseases
11. Treatment of Chronic HEV Infection
11.1. Ribavirin
11.2. Side Effects of Ribavirin
11.3. Ribavirin Treatment Failure
11.4. Pegylated Interferon-α
11.5. Sofosbuvir
12. Hepatitis E Vaccine
13. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Hepatitis E. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 11 March 2023).
- Centers for Disease Control and Prevention. Hepatitis E. 2020. Available online: https://www.cdc.gov/hepatitis/hev/index.htm (accessed on 11 March 2023).
- EASL Clinical Practice Guidelines on hepatitis E virus infection. J. Hepatol. 2018, 68, 1256–1271. [CrossRef]
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.H.; Emerson, S.U. Hepatitis E: An emerging awareness of an old disease. J. Hepatol. 2008, 48, 494–503. [Google Scholar] [CrossRef]
- Wong, D.C.; Purcell, R.H.; Sreenivasan, M.A.; Prasad, S.R.; Pavri, K.M. Epidemic and endemic hepatitis in India: Evidence for a non-A, non-B hepatitis virus aetiology. Lancet 1980, 2, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Lemon, S.M.; Walker, C.M. Enterically Transmitted Non-A, Non-B Hepatitis and the Discovery of Hepatitis E Virus. Cold Spring Harb. Perspect. Med. 2019, 9, a033449. [Google Scholar] [CrossRef] [PubMed]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef]
- Sooryanarain, H.; Meng, X.J. Hepatitis E virus: Reasons for emergence in humans. Curr. Opin. Virol. 2019, 34, 10–17. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Johne, R. Stability of Hepatitis E Virus After Drying on Different Surfaces. Food Environ. Virol. 2022, 14, 138–148. [Google Scholar] [CrossRef]
- Perez-Gracia, M.T.; Suay-Garcia, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017, 27, e1929. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Peron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.J.; Purcell, R.H.; Halbur, P.G.; Lehman, J.R.; Webb, D.M.; Tsareva, T.S.; Haynes, J.S.; Thacker, B.J.; Emerson, S.U. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 1997, 94, 9860–9865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Meng, X.J. Hepatitis E virus: Host tropism and zoonotic infection. Curr. Opin. Microbiol. 2021, 59, 8–15. [Google Scholar] [CrossRef]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B.; Ictv Report, C. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.; Purdy, M.A.; International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2015, 96, 1191–1192. [Google Scholar] [CrossRef]
- Wang, B.; Harms, D.; Yang, X.L.; Bock, C.T. Orthohepevirus C: An Expanding Species of Emerging Hepatitis E Virus Variants. Pathogens 2020, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; van der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Debing, Y.; Ramiere, C.; Dallmeier, K.; Piorkowski, G.; Trabaud, M.A.; Lebosse, F.; Scholtes, C.; Roche, M.; Legras-Lachuer, C.; de Lamballerie, X.; et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J. Hepatol. 2016, 65, 499–508. [Google Scholar] [CrossRef]
- Todt, D.; Gisa, A.; Radonic, A.; Nitsche, A.; Behrendt, P.; Suneetha, P.V.; Pischke, S.; Bremer, B.; Brown, R.J.; Manns, M.P.; et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut 2016, 65, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- van Tong, H.; Hoan, N.X.; Wang, B.; Wedemeyer, H.; Bock, C.T.; Velavan, T.P. Hepatitis E Virus Mutations: Functional and Clinical Relevance. EBioMedicine 2016, 11, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Hepatitis E: Discovery, global impact, control and cure. World J. Gastroenterol. 2016, 22, 7030–7045. [Google Scholar] [CrossRef]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar; Nayak, B.; Ranjith Kumar, C.T.; Surjit, M. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepatitis E Virus. PLoS Pathog. 2016, 12, e1005521. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magden, J.; Takeda, N.; Li, T.; Auvinen, P.; Ahola, T.; Miyamura, T.; Merits, A.; Kaariainen, L. Virus-specific mRNA capping enzyme encoded by hepatitis E virus. J. Virol. 2001, 75, 6249–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, S.U.; Nguyen, H.; Graff, J.; Stephany, D.A.; Brockington, A.; Purcell, R.H. In vitro replication of hepatitis E virus (HEV) genomes and of an HEV replicon expressing green fluorescent protein. J. Virol. 2004, 78, 4838–4846. [Google Scholar] [CrossRef] [Green Version]
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.C.; Saliou, J.M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F.; et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology 2018, 154, 211–223. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Ambardekar, C.; Hu, Z.; Lhomme, S.; Feng, Z. Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog. 2017, 13, e1006417. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.P.; Kasorndorkbua, C.; Halbur, P.G.; Haqshenas, G.; Guenette, D.K.; Toth, T.E.; Meng, X.J. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 2001, 39, 3040–3046. [Google Scholar] [CrossRef] [Green Version]
- Kalia, M.; Chandra, V.; Rahman, S.A.; Sehgal, D.; Jameel, S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009, 83, 12714–12724. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Ambardekar, C.; Lu, Y.; Feng, Z. Distinct Entry Mechanisms for Nonenveloped and Quasi-Enveloped Hepatitis E Viruses. J. Virol. 2016, 90, 4232–4242. [Google Scholar] [CrossRef] [Green Version]
- Perttila, J.; Spuul, P.; Ahola, T. Early secretory pathway localization and lack of processing for hepatitis E virus replication protein pORF1. J. Gen. Virol. 2013, 94, 807–816. [Google Scholar] [CrossRef]
- Rehman, S.; Kapur, N.; Durgapal, H.; Panda, S.K. Subcellular localization of hepatitis E virus (HEV) replicase. Virology 2008, 370, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778. [Google Scholar] [CrossRef] [Green Version]
- Gouttenoire, J.; Pollan, A.; Abrami, L.; Oechslin, N.; Mauron, J.; Matter, M.; Oppliger, J.; Szkolnicka, D.; Dao Thi, V.L.; van der Goot, F.G.; et al. Palmitoylation mediates membrane association of hepatitis E virus ORF3 protein and is required for infectious particle secretion. PLoS Pathog. 2018, 14, e1007471. [Google Scholar] [CrossRef]
- Kannan, H.; Fan, S.; Patel, D.; Bossis, I.; Zhang, Y.J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009, 83, 6375–6382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafrullah, M.; Ozdener, M.H.; Panda, S.K.; Jameel, S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 1997, 71, 9045–9053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanaka, T.; Nishizawa, T.; Yasuda, J.; Okamoto, H. Tumour susceptibility gene 101 and the vacuolar protein sorting pathway are required for the release of hepatitis E virions. J. Gen. Virol. 2011, 92, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Meng, X.J. Structural and molecular biology of hepatitis E virus. Comput. Struct. Biotechnol. J. 2021, 19, 1907–1916. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, e00822-17. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H. Efficient cell culture systems for hepatitis E virus strains in feces and circulating blood. Rev. Med. Virol. 2011, 21, 18–31. [Google Scholar] [CrossRef]
- Okamoto, H. Culture systems for hepatitis E virus. J. Gastroenterol. 2013, 48, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Kenney, S.P.; Meng, X.J. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb. Perspect. Med. 2019, 9. [Google Scholar] [CrossRef]
- Kamani, L.; Padhani, Z.A.; Das, J.K. Hepatitis E: Genotypes, strategies to prevent and manage, and the existing knowledge gaps. JGH Open. 2021, 5, 1127–1134. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S.; Dar, M.Y.; Moecklii, R.; Jameel, S. Hepatitis E and long-term antibody status. Lancet 1993, 341, 1355. [Google Scholar] [PubMed]
- Takahashi, M.; Tanaka, T.; Azuma, M.; Kusano, E.; Aikawa, T.; Shibayama, T.; Yazaki, Y.; Mizuo, H.; Inoue, J.; Okamoto, H. Prolonged fecal shedding of hepatitis E virus (HEV) during sporadic acute hepatitis E: Evaluation of infectivity of HEV in fecal specimens in a cell culture system. J. Clin. Microbiol. 2007, 45, 3671–3679. [Google Scholar] [CrossRef] [Green Version]
- Chandra, N.S.; Sharma, A.; Malhotra, B.; Rai, R.R. Dynamics of HEV viremia, fecal shedding and its relationship with transaminases and antibody response in patients with sporadic acute hepatitis E. Virol. J. 2010, 7, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, G.J.; Mushahwar, I.K.; Chau, K.H.; Gitnick, G.L. Detection of long-lasting antibody to hepatitis E virus in a US traveller to Pakistan. Lancet 1992, 340, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- Baylis, S.A.; Hanschmann, K.M.; Blumel, J.; Nubling, C.M.; Group, H.E.V.C.S. Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: An initial study to evaluate a panel of HEV strains and investigate laboratory performance. J. Clin. Microbiol. 2011, 49, 1234–1239. [Google Scholar] [CrossRef] [Green Version]
- Baylis, S.A.; Blumel, J.; Mizusawa, S.; Matsubayashi, K.; Sakata, H.; Okada, Y.; Nubling, C.M.; Hanschmann, K.M.; Group, H.E.V.C.S. World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerg. Infect. Dis. 2013, 19, 729–735. [Google Scholar] [CrossRef]
- Garson, J.A.; Ferns, R.B.; Grant, P.R.; Ijaz, S.; Nastouli, E.; Szypulska, R.; Tedder, R.S. Minor groove binder modification of widely used TaqMan probe for hepatitis E virus reduces risk of false negative real-time PCR results. J. Virol. Methods 2012, 186, 157–160. [Google Scholar] [CrossRef]
- Zhao, C.; Geng, Y.; Harrison, T.J.; Huang, W.; Song, A.; Wang, Y. Evaluation of an antigen-capture EIA for the diagnosis of hepatitis E virus infection. J. Viral. Hepat. 2015, 22, 957–963. [Google Scholar] [CrossRef]
- Hyams, C.; Mabayoje, D.A.; Copping, R.; Maranao, D.; Patel, M.; Labbett, W.; Haque, T.; Webster, D.P. Serological cross reactivity to CMV and EBV causes problems in the diagnosis of acute hepatitis E virus infection. J. Med. Virol. 2014, 86, 478–483. [Google Scholar] [CrossRef]
- Lenggenhager, D.; Gouttenoire, J.; Malehmir, M.; Bawohl, M.; Honcharova-Biletska, H.; Kreutzer, S.; Semela, D.; Neuweiler, J.; Hürlimann, S.; Aepli, P.; et al. Visualization of hepatitis E virus RNA and proteins in the human liver. J. Hepatol. 2017, 67, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef] [Green Version]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef]
- Hartl, J.; Wehmeyer, M.H.; Pischke, S. Acute Hepatitis E: Two Sides of the Same Coin. Viruses 2016, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Pisano, M.B.; Campbell, C.; Anugwom, C.; Re, V.E.; Debes, J.D. Hepatitis E virus infection in the United States: Seroprevalence, risk factors and the influence of immunological assays. PLoS ONE 2022, 17, e0272809. [Google Scholar] [CrossRef] [PubMed]
- Bouamra, Y.; Gerolami, R.; Arzouni, J.P.; Grimaud, J.C.; Lafforgue, P.; Nelli, M.; Tivoli, N.; Ferretti, A.; Motte, A.; Colson, P. Emergence of autochthonous infections with hepatitis E virus of genotype 4 in Europe. Intervirology 2014, 57, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hakze-van der Honing, R.W.; van Coillie, E.; Antonis, A.F.; van der Poel, W.H. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS ONE 2011, 6, e22673. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Pischke, S.; Manns, M.P. Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology 2012, 142, 1388–1397.e1. [Google Scholar] [CrossRef]
- Aggarwal, R. Clinical presentation of hepatitis E. Virus Res. 2011, 161, 15–22. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S. Aetiology and prognostic factors in acute liver failure in India. J. Viral Hepat. 2003, 10, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Singhal, A.; Panda, S.K.; Acharya, S.K. A 20-year single-center experience with acute liver failure during pregnancy: Is the prognosis really worse? Hepatology 2008, 48, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Manka, P.; Bechmann, L.P.; Coombes, J.D.; Thodou, V.; Schlattjan, M.; Kahraman, A.; Syn, W.K.; Saner, F.; Gerken, G.; Baba, H.; et al. Hepatitis E Virus Infection as a Possible Cause of Acute Liver Failure in Europe. Clin. Gastroenterol. Hepatol. 2015, 13, 1836–1842.e2; quiz e1157–e1838. [Google Scholar] [CrossRef] [Green Version]
- Kamar, N.; Mansuy, J.M.; Cointault, O.; Selves, J.; Abravanel, F.; Danjoux, M.; Otal, P.; Esposito, L.; Durand, D.; Izopet, J.; et al. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am. J. Transplant. 2008, 8, 1744–1748. [Google Scholar] [CrossRef]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef]
- Pischke, S.; Stiefel, P.; Franz, B.; Bremer, B.; Suneetha, P.V.; Heim, A.; Ganzenmueller, T.; Schlue, J.; Horn-Wichmann, R.; Raupach, R.; et al. Chronic hepatitis e in heart transplant recipients. Am. J. Transplant. 2012, 12, 3128–3133. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Selves, J.; Garrouste, C.; Esposito, L.; Lavayssiere, L.; Cointault, O.; Ribes, D.; Cardeau, I.; Nogier, M.B.; et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010, 89, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Stableforth, W.; Thurairajah, P.; Hazeldine, S.; Remnarace, R.; Usama, W.; Farrington, L.; Hamad, N.; Sieberhagen, C.; Ellis, V.; et al. Autochthonous hepatitis E in Southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Bendall, R.P.; Rashid, M.; Ellis, V.; Ali, R.; Ramnarace, R.; Stableforth, W.; Headdon, W.; Abbott, R.; McLaughlin, C.; et al. Host risk factors and autochthonous hepatitis E infection. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1200–1205. [Google Scholar] [CrossRef]
- Choi, J.W.; Son, H.J.; Lee, S.S.; Jeon, H.; Cho, J.K.; Kim, H.J.; Cha, R.R.; Lee, J.M.; Kim, H.J.; Jung, W.T.; et al. Acute hepatitis E virus superinfection increases mortality in patients with cirrhosis. BMC Infect. Dis. 2022, 22, 62. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255. [Google Scholar] [CrossRef]
- Krain, L.J.; Atwell, J.E.; Nelson, K.E.; Labrique, A.B. Fetal and neonatal health consequences of vertically transmitted hepatitis E virus infection. Am. J. Trop. Med. Hyg. 2014, 90, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Fousekis, F.S.; Mitselos, I.V.; Christodoulou, D.K. Extrahepatic manifestations of hepatitis E virus: An overview. Clin. Mol. Hepatol. 2020, 26, 16–23. [Google Scholar] [CrossRef]
- Abravanel, F.; Pique, J.; Couturier, E.; Nicot, F.; Dimeglio, C.; Lhomme, S.; Chiabrando, J.; Saune, K.; Peron, J.M.; Kamar, N.; et al. Acute hepatitis E in French patients and neurological manifestations. J. Infect. 2018, 77, 220–226. [Google Scholar] [CrossRef]
- Dalton, H.R.; Kamar, N.; van Eijk, J.J.; McLean, B.N.; Cintas, P.; Bendall, R.P.; Jacobs, B.C. Hepatitis E virus and neurological injury. Nat. Rev. Neurol. 2016, 12, 77–85. [Google Scholar] [CrossRef]
- Woolson, K.L.; Forbes, A.; Vine, L.; Beynon, L.; McElhinney, L.; Panayi, V.; Hunter, J.G.; Madden, R.G.; Glasgow, T.; Kotecha, A.; et al. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment. Pharmacol. Ther. 2014, 40, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, J.J.; Madden, R.G.; van der Eijk, A.A.; Hunter, J.G.; Reimerink, J.H.; Bendall, R.P.; Pas, S.D.; Ellis, V.; van Alfen, N.; Beynon, L.; et al. Neuralgic amyotrophy and hepatitis E virus infection. Neurology 2014, 82, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eijk, J.J.J.; Dalton, H.R.; Ripellino, P.; Madden, R.G.; Jones, C.; Fritz, M.; Gobbi, C.; Melli, G.; Pasi, E.; Herrod, J.; et al. Clinical phenotype and outcome of hepatitis E virus-associated neuralgic amyotrophy. Neurology 2017, 89, 909–917. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, B.; van der Eijk, A.A.; Pas, S.D.; Hunter, J.G.; Madden, R.G.; Tio-Gillen, A.P.; Dalton, H.R.; Jacobs, B.C. Guillain-Barre syndrome associated with preceding hepatitis E virus infection. Neurology 2014, 82, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Stevens, O.; Claeys, K.G.; Poesen, K.; Saegeman, V.; Van Damme, P. Diagnostic Challenges and Clinical Characteristics of Hepatitis E Virus-Associated Guillain-Barre Syndrome. JAMA Neurol. 2017, 74, 26–33. [Google Scholar] [CrossRef]
- Geurtsvankessel, C.H.; Islam, Z.; Mohammad, Q.D.; Jacobs, B.C.; Endtz, H.P.; Osterhaus, A.D. Hepatitis E and Guillain-Barre syndrome. Clin. Infect. Dis. 2013, 57, 1369–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, T.N.; Lai, S.T.; Lai, J.Y.; Yuen, H. Haemolysis complicating acute viral hepatitis in patients with normal or deficient glucose-6-phosphate dehydrogenase activity. Scand. J. Infect. Dis. 1997, 29, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Chia, L.W.; Kalkur, P.; Lowe, R.; Shannon, M.S. Pathobiology and treatment of hepatitis virus-related thrombocytopenia. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009023. [Google Scholar] [CrossRef]
- Rauff, B.; Idrees, M.; Shah, S.A.; Butt, S.; Butt, A.M.; Ali, L.; Hussain, A.; Irshad Ur, R.; Ali, M. Hepatitis associated aplastic anemia: A review. Virol. J. 2011, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion, O.; Abravanel, F.; Del Bello, A.; Esposito, L.; Lhomme, S.; Puissant-Lubrano, B.; Alric, L.; Faguer, S.; Izopet, J.; Kamar, N. Hepatitis E virus-associated cryoglobulinemia in solid-organ-transplant recipients. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 2178–2189. [Google Scholar] [CrossRef]
- Mallet, V.; Bruneau, J.; Zuber, J.; Alanio, C.; Leclerc-Mercier, S.; Roque-Afonso, A.M.; Kraft, A.R.M.; Couronne, L.; Roulot, D.; Wedemeyer, H.; et al. Hepatitis E virus-induced primary cutaneous CD30(+) T cell lymphoproliferative disorder. J. Hepatol. 2017, 67, 1334–1339. [Google Scholar] [CrossRef]
- Forbes, A.; Woolson, K.L.; Dalton, H.R. Letter: Monoclonal gammopathy of HEV infection. When is it significant?—Authors’ reply. Aliment. Pharmacol. Ther. 2015, 41, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamar, N.; Weclawiak, H.; Guilbeau-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Sallusto, F.; et al. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation 2012, 93, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Schulze Zur Wiesch, J.; Lütgehetmann, M.; Lohse, A.W.; Pischke, S. The Clinical Perspective on Hepatitis E. Viruses 2019, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Raj, J.P.; Kannemkuzhiyil, A.J.; Aluru, J.S.; Thandra, K.C.; Gajendran, M. A Systematic Review of the Extra-Hepatic Manifestations of Hepatitis E Virus Infection. Med. Sci. 2020, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Suneetha, P.V.; Pischke, S.; Schlaphoff, V.; Grabowski, J.; Fytili, P.; Gronert, A.; Bremer, B.; Markova, A.; Jaroszewicz, J.; Bara, C.; et al. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology 2012, 55, 695–708. [Google Scholar] [CrossRef]
- Hansrivijit, P.; Trongtorsak, A.; Puthenpura, M.M.; Boonpheng, B.; Thongprayoon, C.; Wijarnpreecha, K.; Choudhury, A.; Kaewput, W.; Mao, S.A.; Mao, M.A.; et al. Hepatitis E in solid organ transplant recipients: A systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 1240–1254. [Google Scholar] [CrossRef]
- Unzueta, A.; Rakela, J. Hepatitis E infection in liver transplant recipients. Liver Transplant. 2014, 20, 15–24. [Google Scholar] [CrossRef]
- Abravanel, F.; Lhomme, S.; Chapuy-Regaud, S.; Mansuy, J.M.; Muscari, F.; Sallusto, F.; Rostaing, L.; Kamar, N.; Izopet, J. Hepatitis E virus reinfections in solid-organ-transplant recipients can evolve into chronic infections. J. Infect. Dis. 2014, 209, 1900–1906. [Google Scholar] [CrossRef]
- Todesco, E.; Mazzola, A.; Akhavan, S.; Abravanel, F.; Poynard, T.; Roque-Afonso, A.M.; Peytavin, G.; Marcelin, A.G.; Calmus, Y.; Lecuyer, L.; et al. Chronic hepatitis E in a heart transplant patient: Sofosbuvir and ribavirin regimen not fully effective. Antivir. Ther. 2018, 23, 463–465. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jameel, S. Hepatitis E. Hepatology 2011, 54, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- van der Eijk, A.A.; Pas, S.D.; Cornelissen, J.J.; de Man, R.A. Hepatitis E virus infection in hematopoietic stem cell transplant recipients. Curr. Opin. Infect. Dis. 2014, 27, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Fedoravicius, A.; Charlton, M. Abnormal liver tests after liver transplantation. Clin. Liver Dis. 2016, 7, 73–79. [Google Scholar] [CrossRef]
- Aggarwal, A.; Perumpail, R.B.; Tummala, S.; Ahmed, A. Hepatitis E virus infection in the liver transplant recipients: Clinical presentation and management. World J. Hepatol. 2016, 8, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Buffaz, C.; Scholtes, C.; Dron, A.G.; Chevallier-Queyron, P.; Ritter, J.; André, P.; Ramière, C. Hepatitis E in liver transplant recipients in the Rhône-Alpes region in France. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Suneetha, P.V.; Baechlein, C.; Barg-Hock, H.; Heim, A.; Kamar, N.; Schlue, J.; Strassburg, C.P.; Lehner, F.; Raupach, R.; et al. Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transplant. 2010, 16, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Owada, Y.; Oshiro, Y.; Inagaki, Y.; Harada, H.; Fujiyama, N.; Kawagishi, N.; Yagisawa, T.; Usui, J.; Akutsu, N.; Itabashi, Y.; et al. A Nationwide Survey of Hepatitis E Virus Infection and Chronic Hepatitis in Heart and Kidney Transplant Recipients in Japan. Transplantation 2020, 104, 437. [Google Scholar] [CrossRef]
- Versluis, J.; Pas, S.D.; Agteresch, H.J.; de Man, R.A.; Maaskant, J.; Schipper, M.E.; Osterhaus, A.D.; Cornelissen, J.J.; van der Eijk, A.A. Hepatitis E virus: An underestimated opportunistic pathogen in recipients of allogeneic hematopoietic stem cell transplantation. Blood 2013, 122, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Crum-Cianflone, N.F.; Curry, J.; Drobeniuc, J.; Weintrob, A.; Landrum, M.; Ganesan, A.; Bradley, W.; Agan, B.K.; Kamili, S. Hepatitis E virus infection in HIV-infected persons. Emerg. Infect. Dis. 2012, 18, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Juarez, A.; Lopez-Lopez, P.; Frias, M.; Rivero, A. Hepatitis E Infection in HIV-Infected Patients. Front. Microbiol. 2019, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Keane, F.E.; Bendall, R.; Mathew, J.; Ijaz, S. Treatment of chronic hepatitis E in a patient with HIV infection. Ann. Intern. Med. 2011, 155, 479–480. [Google Scholar] [CrossRef]
- Neukam, K.; Barreiro, P.; Macías, J.; Avellón, A.; Cifuentes, C.; Martín-Carbonero, L.; Echevarría, J.M.; Vargas, J.; Soriano, V.; Pineda, J.A. Chronic Hepatitis E in HIV Patients: Rapid Progression to Cirrhosis and Response to Oral Ribavirin. Clin. Infect. Dis. 2013, 57, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Ollier, L.; Tieulie, N.; Sanderson, F.; Heudier, P.; Giordanengo, V.; Fuzibet, J.G.; Nicand, E. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann. Intern. Med. 2009, 150, 430–431. [Google Scholar] [CrossRef]
- Schlevogt, B.; Kinast, V.; Reusch, J.; Kerkhoff, A.; Praditya, D.; Todt, D.; Schmidt, H.H.; Steinmann, E.; Behrendt, P. Chronic Hepatitis E Virus Infection during Lymphoplasmacytic Lymphoma and Ibrutinib Treatment. Pathogens 2019, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.Y.; Zhang, H.C.; Westin, J.; Hosing, C.; Torres, H.A. Hepatitis E Virus Infection in Cancer Patients. Transplant. Cell. Ther. 2022, 28, 788.e781–788.e785. [Google Scholar] [CrossRef]
- le Coutre, P.; Meisel, H.; Hofmann, J.; Röcken, C.; Vuong, G.L.; Neuburger, S.; Hemmati, P.G.; Dörken, B.; Arnold, R. Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation. Gut 2009, 58, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Shimizu, Y.K.; Tanaka, T.; Kuroda, K.; Arakawa, Y.; Takahashi, K.; Mishiro, S.; Shimizu, K.; Moriyama, M. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol. Res. 2007, 37, 113–120. [Google Scholar] [CrossRef]
- Pischke, S.; Peron, J.M.; von Wulffen, M.; von Felden, J.; Höner Zu Siederdissen, C.; Fournier, S.; Lütgehetmann, M.; Iking-Konert, C.; Bettinger, D.; Par, G.; et al. Chronic Hepatitis E in Rheumatology and Internal Medicine Patients: A Retrospective Multicenter European Cohort Study. Viruses 2019, 11, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kounis, I.; Renou, C.; Nahon, S.; Heluwaert, F.; Macaigne, G.; Amil, M.; Talom, S.; Lambare, B.; Charpignon, C.; Paupard, T.; et al. Hepatitis E Virus Infection in Patients with Chronic Inflammatory Bowel Disease Treated with Immunosuppressive Therapy. Pathogens 2023, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Ratho, R.K.; Kumar, S.; Saxena, S.K.; Bora, I.; Thakur, P. Viral Hepatitis E and Chronicity: A Growing Public Health Concern. Front. Microbiol. 2020, 11, 577339. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Abravanel, F.; Lhomme, S.; Rostaing, L.; Kamar, N.; Izopet, J. Protracted fecal shedding of HEV during ribavirin therapy predicts treatment relapse. Clin. Infect. Dis. 2015, 60, 96–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorris, M.; van der Lecq, B.M.; van Erpecum, K.J.; de Bruijne, J. Treatment for chronic hepatitis E virus infection: A systematic review and meta-analysis. J. Viral Hepat. 2021, 28, 454–463. [Google Scholar] [CrossRef]
- Kamar, N.; Del Bello, A.; Abravanel, F.; Pan, Q.; Izopet, J. Unmet Needs for the Treatment of Chronic Hepatitis E Virus Infection in Immunocompromised Patients. Viruses 2022, 14, 2116. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Behrendt, P.; Hofmann, J.; Pageaux, G.P.; Barbet, C.; Moal, V.; Couzi, L.; Horvatits, T.; De Man, R.A.; et al. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin. Infect. Dis. 2020, 71, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Affeldt, P.; Di Cristanziano, V.; Grundmann, F.; Wirtz, M.; Kaiser, R.; Benzing, T.; Stippel, D.; Kann, M.; Kurschat, C. Monitoring of hepatitis E virus RNA during treatment for chronic hepatitis E virus infection after renal transplantation. Immun. Inflamm. Dis. 2021, 9, 513–520. [Google Scholar] [CrossRef] [PubMed]
- van de Garde, M.D.B.; Pas, S.D.; van Oord, G.W.; Gama, L.; Choi, Y.; de Man, R.A.; Boonstra, A.; Vanwolleghem, T. Interferon-alpha treatment rapidly clears Hepatitis E virus infection in humanized mice. Sci. Rep. 2017, 7, 8267. [Google Scholar] [CrossRef] [Green Version]
- Low, E.X.S.; Tripon, E.; Lim, K.; Tan, P.S.; Low, H.C.; Dan, Y.Y.; Lee, Y.M.; Muthiah, M.; Loo, W.M.; Koh, C.J.; et al. Risk factors for ribavirin treatment failure in Asian organ transplant recipients with chronic hepatitis E infection. World J. Hepatol. 2019, 11, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, J. Interferon and Hepatitis B: Current and Future Perspectives. Front. Immunol. 2021, 12, 733364. [Google Scholar] [CrossRef]
- Peters van Ton, A.M.; Gevers, T.J.; Drenth, J.P. Antiviral therapy in chronic hepatitis E: A systematic review. J. Viral Hepat. 2015, 22, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Garrouste, C.; Cardeau-Desangles, I.; Mansuy, J.M.; Weclawiak, H.; Izopet, J.; Rostaing, L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol. Dial. Transplant. 2010, 25, 2792–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haagsma, E.B.; Riezebos-Brilman, A.; van den Berg, A.P.; Porte, R.J.; Niesters, H.G.M. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transplant. 2010, 16, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, F.; Kalligeros, M.; Byrd, K.; Shemin, D.; Mylonakis, E.; Martin, P.; D’Agata, E.M.C. Efficacy and safety of sofosbuvir in the treatment of hep C among patients on hemodialysis: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 14332. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Debing, Y.; Wu, X.; Rice, C.M.; Neyts, J.; Moradpour, D.; Gouttenoire, J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined with Ribavirin. Gastroenterology 2016, 150, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Cornberg, M.; Pischke, S.; Müller, T.; Behrendt, P.; Piecha, F.; Benckert, J.; Todt, D.; Steinmann, E.; Papkalla, A.; von Karpowitz, M.; et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis EThe HepNet SofE pilot study. J. Hepatol. 2020, 73, 696–699. [Google Scholar] [CrossRef]
- van Wezel, E.M.; de Bruijne, J.; Damman, K.; Bijmolen, M.; van den Berg, A.P.; Verschuuren, E.A.M.; Ruigrok, G.A.; Riezebos-Brilman, A.; Knoester, M. Sofosbuvir Add-on to Ribavirin Treatment for Chronic Hepatitis E Virus Infection in Solid Organ Transplant Recipients Does Not Result in Sustained Virological Response. Open. Forum. Infect. Dis. 2019, 6, ofz346. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.F.; Huang, S.J.; Wu, T.; Hu, Y.M.; Wang, Z.Z.; Wang, H.; Jiang, H.M.; Wang, Y.J.; Yan, Q.; et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015, 372, 914–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.A.; Lim, J.K.; Asaga, P.E.P.; Wartel, T.A.; Marti, M.; Yakubu, B.; Rees, H.; Talaat, K.; Kmush, B.; Aggarwal, R.; et al. Hepatitis E vaccine-Illuminating the barriers to use. PLoS Negl. Trop. Dis. 2023, 17, e0010969. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, P.; Lin, H.; Hao, X.; Liang, Z. Hepatitis E virus: Current epidemiology and vaccine. Hum. Vaccin. Immunother. 2016, 12, 2603–2610. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songtanin, B.; Molehin, A.J.; Brittan, K.; Manatsathit, W.; Nugent, K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses 2023, 15, 1389. https://0-doi-org.brum.beds.ac.uk/10.3390/v15061389
Songtanin B, Molehin AJ, Brittan K, Manatsathit W, Nugent K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses. 2023; 15(6):1389. https://0-doi-org.brum.beds.ac.uk/10.3390/v15061389
Chicago/Turabian StyleSongtanin, Busara, Adebayo J. Molehin, Kevin Brittan, Wuttiporn Manatsathit, and Kenneth Nugent. 2023. "Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations" Viruses 15, no. 6: 1389. https://0-doi-org.brum.beds.ac.uk/10.3390/v15061389