1. Introduction
Regeneration of bone critical defects is still a challenge in the orthopedic field. Local treatment with bone morphogenetic protein (BMP-2) incorporated in different biomaterial scaffolds has demonstrated to be efficient to induce new bone formation for critical bone defect in several animal models. Nowadays, collagen sponges loaded with recombinant BMP-2 are clinically available as bone graft substitutes for the treatment of nonunion and critical-sized bone defects. Although BMP-2 is a potent osteogenic agent, a controlled release profile is required for safety and efficacy. Thus, the scaffold to fill the bone defect should not only be designed to act as support and guide for tissue growth, but also to control the release rate of active substances. Although many materials and structures have been proposed to construct these scaffolds, the control of the release rate has not always been taken into account. In fact, in some cases the protein is incorporated in the material by incubation and, unless a material-protein interaction occurs, a burst release at an early period would be expected. Consequently, a high dose of BMP-2 would be in blood circulation, leading to a high risk of side effects and, at the same time, a significant loss of the protein in the site of action. In addition, some authors showed that BMPs in these cases might also stimulate bone resorption due to the high dose of BMP-2 associated to uncontrolled release [
1]. To minimize the osteoclastic effect of BMPs, some authors proposed the addition of an anti-catabolic agent. The most frequently studied combination has been BMP-2 with bisphosphonates as anti-resorption agents such as alendronate [
2,
3] and zolendronate [
4]. The results indicated good bone regeneration with improved bone quality and mineralization in different localizations compared to BMP-2 alone [
5,
6].
Among the several natural polymeric scaffolds prepared for bone regeneration, chitosan is a biomaterial frequently used for this purpose. In a recent extensive review [
7] based on chitosan (CHT) applied in bone tissue regeneration, different advantageous aspects were showed and discussed such as biocompatibility, capacity for BMPs sustained release, improvement of cell proliferation, and increase of in vitro and in vivo differentiation and mineralization. However, bone graft materials to simulate bone structure, e.g., collagen, the major ECM of bone tissue, and hydroxyapatite (HAP), a mineral component of the bone, have also been widely studied [
6,
8,
9,
10,
11,
12,
13,
14].
Although the aforementioned studies indicated positive results, the mentioned strategies applied in osteoporosis (OP) conditions have not always been effective [
15]. According to recent reports, the prolonged subcutaneous administration of alendronate and the low level of estrogen in OP alters the evolution of calvarial bone repair due to estrogen, Transforming Growth Factor beta 1 (TGF-β1), and α-estrogen receptor (α-ER) interaction [
16]. Menopausal women are the population most affected by this disease due to estrogen deficiency. Previous reports revealed that local implantation of scaffolds loaded with combinations of BMP-2 and 17β-estradiol formulated in microspheres of polylactic acid (PLA) or PLGA, in rat calvaria critical defects increased the bone repair in OP rats, but the new bone that refilled the defect was less mineralized compared to non-OP groups [
17,
18].
As some biomaterials may promote bone regeneration, controlled release of the active substances is required for efficient and safe bone regeneration [
19,
20]. In this study, we propose a loaded BMP-2 and 17β-estradiol sandwich-like system, comprising two polymeric external films and a core of a biocomposite hydrogel containing microspheres, to provide sustained release of active substances. The core system composed of CHT, collagen, HAP nanoparticles (nano-HAP), polyethylene glycol (PEG-400) and 2-Hidroxipropil γ-Ciclodextrin HP-γ-CD was previously characterized in terms of composition, rheological behavior and mass-transfer using RITC-dextran as macromolecule model and 17β-estradiol in microspheres [
21]. In the present study, we aim to study the influence of the release rate of 17β-estradiol on the osteogenic effect induced by BMP-2 released from PLGA microspheres within core system after sandwich-like system implantation in a OP rats critical size defect. Therefore, 17β-estradiol was incorporated into the system in 3 forms: free and dispersed in the core, encapsulated in microspheres prepared with a mixture of PLA and PLGA dispersed within the hydrogel and lastly encapsulated in the PLGA films shell prepared by electrospinning technique.
2. Materials and Method
2.1. Materials
PLGA 75:25 (Resomer® RG755-S), PLGA 50:50 (Resomer® RG504), PLGA 85:15 (Resomer® RG858-S), and PLA (Resomer® RG203-S) were supplied by Evonic Industries (Darmstadt, Germany). Chitosan (Protasan® UP-CL-213) was purchased from NovaMatrix (Sandvika, Norway). 2-Hidroxipropil γ-Ciclodextrin (CAVASOL® W8 HP), was supplied by Wacker Chemical (Burghausen, Germany). The bovine collagen type I was purchased from CellSystems Biotechnologie (Vertrieb GmbH, Germany). Riboflavin (RB), Poly(ethylene glycol) 400, Poly(vinyl alcohol) (PVA, Mw 33–70 kDa; 87–90% hydrolyzed), 17β-estradiol, and all the other reagents were purchased from Sigma-Aldrich, (St. Louis, MO, USA). The recombinant human bone morphogenetic protein 2 (BMP-2) was bought from Biomedal Life Sciences (Sevilla, Spain). Citrate-coated carbonated apatite nanoparticles (nano-HAP) were kindly donated (Jaime Gómez-Morales, PhD, Laboratory of Crystallographic Studies, CSIC, Granada, Spain).
2.2. Microspheres Preparation and Characterization
The BMP-2 microspheres were prepared by the double emulsion method (w/o/w) previously described [
22]. Briefly, 200 µL of an aqueous solution (0.2% PVA) of BMP-2 (260µg) was emulsified with 1 mL of a PLGA mixture (150 mg) of RG504 and RG858 [4:1] in methylene chloride (DCM) by vortexing 1 min (position 10, Genie
® Industries 2, Sciencies Industries Inc. USA). Then, this emulsion was poured into 10 mL of 0.2% PVA solution vortexed 15 s, then poured into 100 mL of 0.1% PVA and kept under magnetic stirring for 1 h for solvent evaporation.
The 17β-estradiol microspheres were prepared by a modified solvent evaporation method previously described [
17]. Briefly a mixture of 17β-estradiol (4mg), PLA-S RG203-S (160 mg) and PLGA RG858 (40 mg) dissolved in 0.6 mL of DCM:Methanol (DCM:MeOH) (80:20) was emulsified with 4 mL of 1% PVA aqueous solution by vortexing 1 min (position 10), and then added to 100 mL of 0.16% PVA solution, under magnetic stirring for organic solvent evaporation (1 h).
Both type of microspheres were collected by filtration (Pall Corporation, pore size 45 µm, Sigma-Aldrich, USA), lyophilized, and stored at 4 °C until use.
Microspheres were characterized in terms of size (Mastersizer 2000, Malver Instruments, Malvern, UK) and morphology (SEM, Jeol JSM-6300, Tokyo, Japan). To determine the BMP-2 encapsulation efficiency and to carry out the BMP-2 release assays, some batches were prepared with
125IBMP-2. The BMP-2 was labeled with
125INa (Perkin-Elmer) by the iodogen method [
23]. The content of 17β-estradiol in the microspheres was determined spectrophotometrically at λ = 280 nm previous dissolution in a mix of DCM:MeOH (80:20).
To determine the solubility of 17β-estradiol in the polymer matrix of the microspheres, differential scanning calorimetry (DSC 025, TA Instruments, New Castle, DE, USA) was performed. 17β-estradiol and lyophilized microspheres were analyzed after drying in an oven at 37 °C overnight. In addition, samples of polymer blends (RG 203-S and RG 858, 4:1) and samples of the polymer blend with excess 17β-estradiol (8.5%) were dissolved in DCM:MeOH (80:20) and maintained in a hood for 24 h. Then, samples were placed 48 h more in a vacuum desiccator to complete the evaporation of the organic solvent. The analysis of all samples was performed with the same thermal program in two thermal cycles under a nitrogen atmosphere (50 mL/min). In the first cycle, temperature was increased to 40 °C (10 °C/min) and then cooled to −20 °C (5 °C/min) to avoid possible water interference. Once the samples were stabilized, they underwent a final heating cycle from −20 °C to 270 °C (10 °C/min).
2.3. Fabrication and Characterization of the Film
The film was fabricated by a previously described electrospinning method [
24]. Briefly, 7 mg of 17β-estradiol and 300 mg of a mixture of PLGAs, RG755-S and RG858 [4:1] were dissolved in 2 mL of hexafluoroisopropanol (Sigma-Aldrich, Steinheim, Germany) and electrospun at 7kV; flow rate of 3.0 mL/h and 10 cm of distance from the collector.
The film quality was checked in terms of porosity, thickness and fiber diameter using helium pycnometer (AccuPyc 1330, Micromeritics, Norcross, GA, USA), stereo microscopy (Leica M205C, Leica Las, v3 sofware), and SEM (Jeol, JSM-6300, Tokyo, Japan), respectively.
2.4. Core System Preparation and Characterization
To prepare the core system, approximately 20 mg of microspheres were dispersed in 50 µL of the hydrogel composed by a mixture of collagen type I (5 mg/mL), HP-γ-CD (34 mg/mL), RB (0.4 mg/mL), CHT (5 mg/mL), PEG-400 (150 mg/mL), and 5 mg of nano-HAP. Then, the hydrogel was cross-linked with 5%
w/
w TPP sterile aqueous solution (0.5 µL/µL of hydrogel) and visible light blue at 468 nm (Dental device) for 3 min [
21]. The dose of BMP-2 was 6 µg in microspheres and the total 17β-estradiol dose was 200 µg in three different forms: electrospun films, microspheres, or dispersed into the gel.
The quality of the core system was checked according to its rheological characteristics and porosity previously described [
21]. In addition, water uptake and mass loss assays were carried out by incubation of aliquots of 300 µL of the core system in 5 mL of sterile MilliQ water (37 °C) under orbital agitation (25 rpm). At specific times, six samples were withdrawn, we then removed excess water, weighed, and freeze-dried the samples. Then, three samples were visualized by SEM (Jeol JSM-6300) to see the evolution of the internal structure after incubation. The other three samples were used to record the dried weight and calculate the percentage of mass loss and water uptake, applying Equations (1) and (2), respectively, where
W0 is the initial weight of the sample and
Ww and
Wd are the weights of the wet and dried sample, respectively, at the different times tested.
To test the syringeability of the core system two syringes of 1 mL were loaded with a suspension of microspheres of 17β-estradiol in the hydrogel, up to 0.5 mL. Then, 4 doses of 50 µL each were unloaded from both syringes assayed for fluidity through a 20G needle and dose uniformity. For this, the discharged samples were lyophilized, and the 17β-estradiol content evaluated by spectrophotometry at 280 nm, after dissolution in DCM: MeOH (80:20).
2.5. In Vitro Release Assays
BMP-2 in vitro release assays were carried out by incubating an amount of 125I-BMP-2 microspheres and an amount of core system with the same amount of 125I-BMP-2 microspheres in sterile MilliQ water at 37 °C and 25 rpm. The amount of BMP-2 released was calculated by measuring the radioactivity of supernatant samples with a gamma counter (Cobra® II, Packard).
The in vitro release of 17β-estradiol from the different formulations (dispersed in the core system, microspheres, microspheres incorporated to the core system, and electrospun films) was carried out at 37 °C and 25 rpm using two release media: an aqueous solution of sodium lauryl sulfate (SLS) 1% [
25] and MeOH:water (50:50) [
26,
27]. The released 17β-estradiol was measured in the supernatant using the spectrophotometric method. The effective diffusion coefficient,
Deff in the matrix of the microspheres and the mass transfer coefficient of the drug in the boundary layer
h, were calculated according to the non-steady-state Fick law, as previously described in detail [
21]. Whether or not the released fraction of 17β-estradiol from the microspheres dispersed in the core system was analyzed, and Equations (3)–(6) were applied for
Deff and
h calculation.
where
Mt and
M∞ are the total mass of drug released to the media at time
t and at the end of the experiment, respectively. The
βns are the infinite roots (eigenvalues) of the Equation (4):
L is the dimensionless mass transfer Biot number Equation (5):
For large values of
L, the roots of Equation (4) are multiples of the number
pi and Equation (3) can be simplified in the Equation (6), that is, a simplified solution of non-steady-state Fick law:
As stated previously, to minimize the residual sum of squares, “genetic algorithms” already implanted in R software (R Foundation for Statistical Computing, version 3.6.1., 2019, Vienna, Austria) were used [
21].
2.6. Animal Experiments
All animal experiments were carried out in conformity with the European Directive (2010/63UE) on Care and Use in Experimental Procedures. Furthermore, the animal protocols were approved on 5 November 2014 by the Ethics Committee for Animal Cares of the University of La Laguna (CEIBA) with identification code CEIBA2014-0128. All surgical procedures were made under aseptic conditions.
2.6.1. Animal Models
Forty female adult Sprague-Dawley rats approximately 12 weeks old, weighing 200–250 g, were divided in 4 groups of 10 each. The experimental osteoporosis was induced in 3 groups by three different protocols, OVX, chronic administration of DEX and OD. The forth group was the sham, non-osteoporotic control group (non-OP). The bilateral ovariectomy was carried out under isoflurane anesthesia, via dorsal approach to the animals of OVX and OD groups. Analgesia consisted in buprenorphine administered subcutaneously (0.05 mg/kg) before surgery and paracetamol (70 mg/100mL) in the water, for 3 days post-surgery. The DEX group received 0.3 mg/kg body weight of dexamethasone-21-isonicotinate (Deyanil retard, Fatro Ibérica, Barcelona, Spain) administered subcutaneously once ievery two weeks [
28] up to the time of euthanasia. Then, two weeks after the ovariectomy, the rats of group OD were chronically treated with DEX as the DEX group. The 40 rats were sacrificed after 12 weeks and the calvaria and femurs were extracted to be histologically analyzed. The results of these analyzes were used to evaluate the 3 protocols tested to induce OP.
2.6.2. Animals Groups
Fifty female Sprague-Dawley rats (12 weeks old), weighing 200–250 g, were divided into 2 groups of 25 each: OP and non-OP. The rats of the OP group were ovariectomized and the rats of the non-OP group underwent similar surgery but the ovaries were not resected. Twelve weeks post-surgery, 8 mm critical size cranial defects were created surgically with a trephine burr in the rats under isoflurane and the systems were placed into the defects [
20]. Analgesia treatment was administrated.
Female rats were divided into 5 groups of 10 rats each—5 OP and 5 non-OP—and the applied regenerative treatment is reflected in
Table 1. The implantation of the systems was carried out following a two steps procedure. First, a layer of film (bottom film), previously soaked in the blood produced during the surgery, was placed in the defect then 50 µL of the hydrogel mixed with the microspheres and partially cross-linked with UV light, was discharged. Second, the hydrogel was completely cross-linked by dripping 25 µL of sodium tripolyphosphate (TPP) forming the core system, after 5 min, a second layer of film (soaked in blood) was placed on the top, like a sandwich, and the wound was then closed.
2.6.3. 125I-BMP-2 in Vivo Release Assay
The BMP-2 release kinetics was monitored periodically by measuring the remaining
125I-BMP-2 at the rat calvarial defect site (
n = 5) using an external probe-type gamma counter (Captus
®, Capintec Inc., Ramsey, NJ, USA), as previously described and validated [
29].
2.7. Rat Mesenchymal Stem Cells (rMSCs) Osteogenic Differentiation
The rMSCs were obtained by centrifugal isolation as previously described [
30] from the bone marrow of the femur of OVX female Sprague-Dawley rats. Briefly, the cells were resuspended in high glucose DMEM (HyClone
® Utah) supplemented with 10% fetal bovine serum (Biowest, South America Origin), 1% penicillin–streptomycin (PAA, Pasching, Austria), and 2 mM
l-Glutamine stable (Biowest, France) (Complete Culture Medium, CCM). Then, cells were cultured in flasks of 75 cm
2 and subcultured by incubating at 37°C and 5% CO
2. The culture medium was changed every 2–3 days.
To test the osteogenic differentiation, 50,000 cells (passage 2) in 20 μL of CCM were added over aliquots of 300 μL of the core system (hydrogel with microspheres) with and without nano-HAP and incubated at 37 °C and 5% CO2 for 1.5 h for cell adhesion. The homogeneous cell distribution was checked by light microscopy. Afterwards, 500 μL of CCM were added to each well, after 3 days incubation the medium was changed to CCM supplemented with 10 mM β-glycerol phosphate, 10–7 M dexamethasone and 50 μM ascorbate-2-phosphate. At 7, 14, and 21 days of culture, three wells of each time point were washed (2 times) with Hank’s balanced salt solution (HBSS 1x) and cooled at 4 °C. Then, 500 µL of 0.1 M buffer Tris-HCl, 0.1M NaCl, and 0.05 M MgCl2 (pH = 9.2–9.5) containing Nitro blue tetrazolium chloride (NBT, Roche Diagnostics, Mannheim, Germany) and 5-Bromo-4-chloro-3-indolyl phosphate (BCIP, Roche Diagnostics, Mannheim, Germany) were added and incubated at 37 °C and 5% CO2 under soft agitation for 1.5 h. Then, the NBT/BCIP was removed and the cells were fixed with a solution of 3.7–4% p-formaldehyde buffered to pH = 7.0 (Panreac®, Barcelona, Spain) during 30 min. After this, the formaldehyde was removed, and the wells were washed 3 times with HBBS 1x. Immediately after this, cells were visualized by stereo microscopy (Leica M205C, Leica Las, v3 software). In addition, samples were dehydrated in a graded series of ethanol before being embedded in Paraplast® and microtome (Shandon Finesse 325, Thermo Fisher Scientific, Madrid, Spain) sections were observed by light microscopy (LEICA DM 4000B, Barcelona, Spain).
2.8. Histology, Immunohistochemical, and Histomorphometrically Evaluation
First, to check the osteoporotic-like condition, 12 weeks after the 12 rats undergone the different protocols were sacrificed and the femurs and calvaria were analyzed. The femurs and calvaria were fixed (4% paraformaldehyde solution), decalcified in Histofix
® Decalcifier (Panreac, Barcelona, Spain) and prepared for histological analysis as previously described [
20,
22].
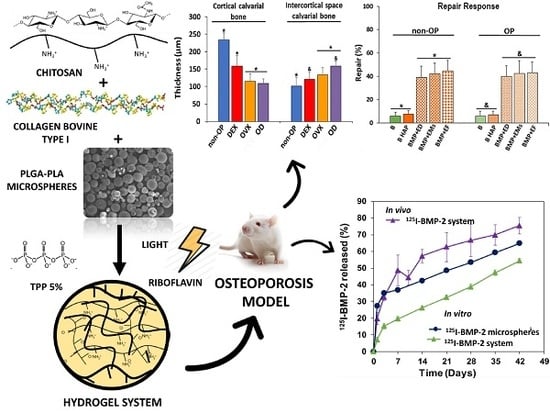
Bone morphology was analyzed by hematoxylin–erythrosin staining. The histomorphometric analyze was carried out in femurs by measuring the following parameters; thickness of the cortical bone (Ct.Wi) and number (Tb.N), width (Tb.Wi) and separation (Tb.Sp) of the trabeculae in cancellous bone. In the calvaria bone, the histomorphometric analysis was carried out by measuring the following parameters, cortical bone thickness (CBT) and intercortical space thickness (IST) occupied by trabecular bone in transversal sections of calvaria.
To determine the capacity of the bone active substances, so as to regenerate the critical size defect practiced in the calvaria of the rats, samples of the 10 groups of 5 rats each were examined.
Samples were processed as previously described [
22]. New bone formation was identified by hematoxylin–erythrosin staining. Bone mineralization was assessed with VOF trichrome stain, in which red and brown staining indicates advanced mineralization, whereas less mineralized, newly formed bone stains blue [
31]. Sections were analyzed by light microscopy (LEICA DM 4000B, Barcelona, Spain). Computer-based image analysis software (Leica Q-win V3 Pro-Image Analysis System, Barcelona, Spain) was used to evaluate all sections. A region of interest (ROI) within the defect (50 mm
2) for quantitative evaluation of new bone formation was defined. New bone formation was expressed as a percentage of repair with respect to the original defect area within the ROI. From the total bone repair, the areas of mature bone (MB) and immature bone (IB) were determined, and the MB/IB ratio for each experimental group as well as between non-osteoporotic and osteoporotic-like animals was calculated.
For immunohistochemical analysis, sections were deparaffined and rehydrated in Tris-buffered saline (TBS) (pH 7.4, 0.01 M Trizma base, 0.04 M Tris hydrochloride, 0.15 M NaCl), which was used for all further incubations and rinse steps. Sections were incubated in citrate buffer (pH 6) at 90°C for antigen retrieval, followed by incubation in 0.3% hydrogen peroxide in TBS buffer for 20 min. After a rinse step, sections were blocked with 2% FBS in TBS–0.2% Triton X-100 (blocking buffer). The indirect immunohistochemical procedure was carried out by incubating the sections with osteocalcin (OCN) polyclonal antiserum (1/100) (Millipore, Barcelona, Spain) in blocking buffer overnight at 4 °C. Sections were rinsed three times, then incubated with biotin-SP-conjugated donkey anti-rabbit F(ab) fragment (1/200) (Millipore, Barcelona, Spain) in blocking buffer for 1 h followed, after another rinse step, by incubation in peroxidase-conjugated streptavidin (1/300) (Millipore, Barcelona, Spain) for 1 h. Peroxidase activity was revealed in Tris–HCl buffer (0.05 M, pH 7.6) containing 0.05% of 3,3′-diaminobenzidine tetrahydrochloride (Sigma, Poole, UK) and 0.004% hydrogen peroxide. Reaction specificity was confirmed by replacing the specific antiserum with normal serum or by pre-adsorption of the specific antiserum with the corresponding antigen.
OCN staining was evaluated using computer-based image analysis software (ImageJ, NIH, Bethesda, MD, USA). OCN staining was measured by applying a fixed threshold to select for positive staining within the ROI. Positive pixel areas were divided by the total surface size (mm2) of the ROI. Values were normalized to those measured from blank scaffolds and are reported as relative staining intensities.
Statistical analysis was performed with SPSS.25 software. We compared the distinct treatments by means of a one-way analysis of variance (ANOVA) with a Tukey multiple comparison post-test. Significance was set at p < 0.05. Results are expressed as means ± SD.
4. Discussion
In the present study, a BMP-2-17β-estradiol hydrogel system, with porosity of approximately 72%, was evaluated for regeneration of a critical size defect in rat calvaria. Although the system had already been characterized in terms of rheological behavior, porosity, interactions between components, mass transfer parameters, and cell viability, here the good injectability of the system was showed, and its characterization has been completed by testing the water uptake and mass loss as well as differentiation studies in cultures of osteoporotic rMSCs. In vitro release profiles of 17β-estradiol in two media and the in vitro and in vivo release profile of BMP-2 were also analyzed.
The osteogenic differentiation of osteoporotic rMSCs seeded on the system was assessed in order to test the effect of the incorporation of the nano-HAP on the cell behavior. The results showed greater ALP activity and a greater number of differentiated cells in the systems with nano-HAP. Therefore, nano-HAP systems were subsequently used in the in vivo experiments.
First, a histological evaluation of the femur and calvaria of rats suffering the three treatments for osteoporosis induction was carried out. OP is a systemic bone disease characterized by the increase of bone porosity, loss of bone mass and changes in the microstructure of the skeletal. Consequently, the OP population has an increased risk of fracture.
Despite the high number of OP studies and the several publications dedicated to tissue repair in non-OP specimens, very few reports devoted to bone defect regeneration in OP have been published. As OP might be primary post-menopausal or secondary, due to corticoid chronic administration, three animal models were used for OP induction: OVX, chronic glucocorticoid treatment, and the combination of both. In previous reports, a combination OD rat model was used. However, the high deterioration observed in the animals, the risk of induced additional disorders on the skeletal and the fact that bone loss reverses after corticoid stop [
33] justify the bone histological study of the different treatment, in order to simplify the model and improve animal welfare. In general, OP condition is established through the analysis of long bones and lumbar spine but few data of the effect on the calvaria of the animals used as OP models are available [
34]. Most of the publications on the regeneration of calvaria critical size defect in OP animals do not report the effects of OP in the calvaria [
35,
36]. In the present study, the data comparing the response of the femurs and calvarias to the three treatments revealed that the effect of OVX was similar to OD combination, consequently the fabricated system was tested in OVX rats.
As some authors have observed a delay in bone consolidation of OVX rats [
37] and as this combination of BMP-2 and 17β-estradiol, formulated in microspheres, when applied to a critical calvaria defect, improved bone healing in OP rats, but the new bone was less mineralized [
17,
18], we tried to prolong the release of active substances to cover this delay. The drugs were incorporated to the hydrogel system pre-encapsulated in microspheres for prolonged controlled release. To reduce the release rate of the active substances the microspheres were prepared with a mixture of polymers, 25% of the RG 858 was incorporated to the RG 504 for BMP-2 microspheres as well as to the RG203-S for 17β-estradiol microspheres. RG 858 is a PLGA 85:15, of high molecular weight with a degradation rate lower than that of RG 504 and RG203-S. The BMP-2 release profiles showed a two-phase behavior with a weak burst effect that coincides with the period in which the system loses mass and uptakes a high amount of water. However, the burst effect of BMP-2 that can be seen from the microspheres was damped by the hydrogel, probably due to the interaction of the protein with the chemical groups of the HAP [
6,
13]. Afterwards, the second phases were practically parallel, which indicated a mass transfer process controlled by the access of water inside the microspheres, dissolution and diffusion of the protein throughout the porous of the polymeric matrix.
The release profile of 17β-estradiol as liposoluble drug in a MeOH:water (60:40) release medium was previously characterized [
21]. By contrast, here two release media were used, MeOH:water (50:50) and an aqueous solution of SLS, because we suspected that the solvent affected the release rate of the lipophilic substance. In fact, the release of 17β-estradiol in MeOH:water was fast regardless of the formulations. However, the in vitro release of 17β-estradiol was formulation-dependent when an aqueous medium was used; a high decrease of the release rate of the drug from the microspheres was observed. As DSC results indicated, 17β-estradiol formed a solid solution in the microspheres, which indicates that the release process takes place by molecular diffusion of 17β-estradiol within the microspheres, governed by the partition coefficient, and consequently the aqueous medium dissolved it very slowly. In addition, the higher estimated values of
Deff in the MeOH:water compared with the aqueous medium, confirmed the dependency on the release media. The calorimetric analysis of the electrospun sheet was not carried out because the amount of 17β-estradiol would have had to be increased to be detected and its characteristics would have been modified. Although 17β-estradiol is expected to also be dissolved in the polymer of the film, the large specific surface area that microfibers expose to the medium causes the drug to release rapidly. Similarly, 17β-estradiol dispersed in the hydrogel was very little retained. Obviously, neither media are physiological, but it seems more correct to use an aqueous medium to predict release in vivo. Although, with reservations, one would also expect a slightly faster release rate in vivo as the biological components present in the tissue could accelerate the drug release from the system.
Despite the beneficial role of the nano-HAP controlling the BMP-2 burst effect as well as its positive effect on the proliferation and osteogenic differentiation of rMSC, which justifies the use of nano-HAP in the system, the reparative effect of the blank scaffolds with and without nano-HAP was not enough to be considered useful. Unlike that observed in this study, other authors showed better bone repair in different bone defects practiced in osteoporotic goats implanted with a system of type I collagen containing nano-HAP than without [
38]. By contrast, another study [
6] found, as in the present study, that the use of nano-HAP and calcium sulfate bone substitute scaffolds in rat critical calvaria defect showed no effect on repair and mineralization at 8 and 12 weeks with respect to the empty defect. In both studies, the systems were loaded with BMP-2 combined with 17β-estradiol or zolendronic acid [
6] that might abolish the effect of the HAP observed in vitro.
Although previously our group reported [
17,
18] a better result in bone regeneration of OP animals combining BMP-2 and 17β-estradiol, in this study, the repair effect observed has been similar to that observed in non-OP groups. The three combinations of BMP-2 with 17β-estradiol in each of the three formulations used showed the same effect at 12 weeks. However, the ratios of mature and immature bone in normal and osteoporotic animals showed significant differences, indicating that the quality of the repaired bone, at least after 12 weeks, was better in normal animals.
Although these results coincide with previous work, in the present study it seems that the mineralization of the bone formed slightly improved in the OP animals. The difference in the relative osteocalcin expression was not statistically significant. These results might suggest that a longer release of BMP-2 together with the composition of the system, presence of collagen and nanoHAP favor the mineralization process. In any case, it would be necessary in future to conduct studies aimed at discerning the role of each of these components in the process. However, we have not been able to reproduce the positive effect of 17β-estradiol combined with BMP-2 in a hydrogel composed of Pluronic, Tetronic and, cyclodextrin, with other scaffolds of different composition [
18]. In addition, according to the present study, the fact that the different release profiles of 17β-estradiol had no effect on the repair of the defect indicates that 17β-estradiol, when applied locally, and regardless of the release rate (available dose) and of the obvious role that it plays on bone remodeling, does not justify its inclusion as active substance in the repair of bone defects neither in normal animals nor in osteoporotic ones. Therefore, new strategies and alternative drugs are currently being designed trying to accelerate mineralization of new bone in OP groups.