-
 Praziquantel Fifty Years on: A Comprehensive Overview of Its Solid State
Praziquantel Fifty Years on: A Comprehensive Overview of Its Solid State -
 Piperacillin/Tazobactam Co-Delivery by Micellar Ionic Conjugate Systems Carrying Pharmaceutical Anions and Encapsulated Drug
Piperacillin/Tazobactam Co-Delivery by Micellar Ionic Conjugate Systems Carrying Pharmaceutical Anions and Encapsulated Drug -
 Lipid-Based Nanotechnology: Liposome
Lipid-Based Nanotechnology: Liposome -
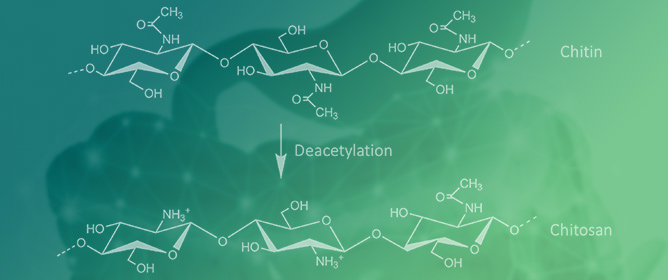 Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers
Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers -
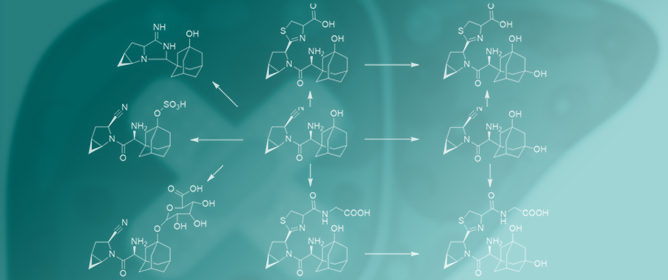 Evaluation of the Drug-Induced Liver Injury Potential of Saxagliptin through Reactive Metabolite Identification in Rats
Evaluation of the Drug-Induced Liver Injury Potential of Saxagliptin through Reactive Metabolite Identification in Rats
Journal Description
Pharmaceutics
Pharmaceutics
is a peer-reviewed, open access journal on the science and technology of pharmaceutics and biopharmaceutics, and is published monthly online by MDPI. The Spanish Society of Pharmaceutics and Pharmaceutical Technology (SEFIG), Pharmaceutical Solid State Research Cluster (PSSRC), Academy of Pharmaceutical Sciences (APS) and Korean Society of Pharmaceutical Sciences and Technology (KSPST) are affiliated with Pharmaceutics and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology & Pharmacy) / CiteScore - Q1 (Pharmaceutical Science)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 14.2 days after submission; acceptance to publication is undertaken in 3.6 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Companion journal: Future Pharmacology
Impact Factor:
5.4 (2022);
5-Year Impact Factor:
6.0 (2022)
Latest Articles
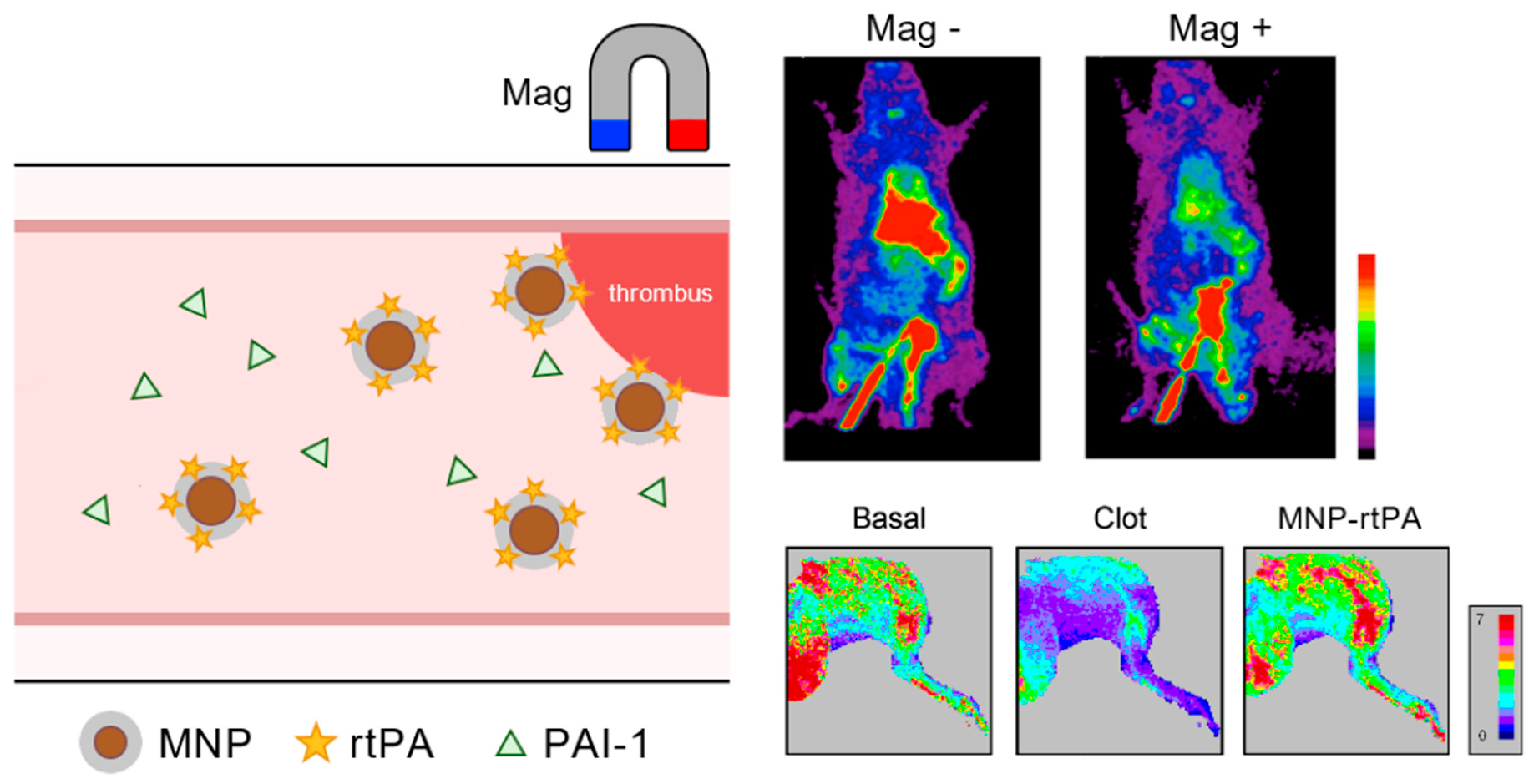
Targeted Thrombolysis with Magnetic Nanotherapeutics: A Translational Assessment
Pharmaceutics 2024, 16(5), 596; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050596 (registering DOI) - 27 Apr 2024
Abstract
Plasminogen activators, such as recombinant tissue-type plasminogen activators (rtPAs), while effective in treating thromboembolic diseases, often induce hemorrhagic complications due to non-specific enzyme activities in the systemic circulation. This study evaluated the targeting efficiency, efficacy, biodistribution, and potential toxicity of a rtPA covalently
[...] Read more.
Plasminogen activators, such as recombinant tissue-type plasminogen activators (rtPAs), while effective in treating thromboembolic diseases, often induce hemorrhagic complications due to non-specific enzyme activities in the systemic circulation. This study evaluated the targeting efficiency, efficacy, biodistribution, and potential toxicity of a rtPA covalently attached to chitosan-coated magnetic nanoparticles (chitosan-MNP-rtPA). The thrombolytic activity of a chitosan-MNP-rtPA was preserved by protection from an endogenous plasminogen activator inhibitor-1 (PAI-1) in whole blood and after circulation in vivo, as examined by thromboelastometry. Single-photon emission computed tomography (SPECT) demonstrated real-time retention of a 99mTc-MNP-rtPA induced by magnet application in a rat embolic model; an 80% reduction in rtPA dosage for a chitosan-MNP-rtPA with magnetic guidance was shown to restore blood flow. After treatment, iron deposition was observed in the reticuloendothelial systems, with portal edema and neutrophil infiltration in the liver at a ten-fold higher dose but not the regular dose. Nevertheless, no liver or renal toxicity was observed at this higher dose. In conclusion, the liver may still be the major deposit site of rtPA nanocomposites after targeted delivery; chitosan-coated MNPs are potentially amenable to target therapeutics with parenteral administration.
Full article
(This article belongs to the Special Issue Recent Advances in Biomedical Applications of Magnetic Nanomaterials)
►
Show Figures
Open AccessArticle
Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes
by
Letícia Kakuda, Patrícia M. B. G. Maia Campos and Wanderley P. Oliveira
Pharmaceutics 2024, 16(5), 595; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050595 (registering DOI) - 27 Apr 2024
Abstract
Encapsulation and drying technologies allow the engineering of innovative raw materials from plant biodiversity, with potential applications in pharmaceutical and cosmetic fields. Lipid-based nanoencapsulation stands out for its efficiency, ease of production, and versatility in encapsulating substances, whether hydrophilic or lipophilic. This work
[...] Read more.
Encapsulation and drying technologies allow the engineering of innovative raw materials from plant biodiversity, with potential applications in pharmaceutical and cosmetic fields. Lipid-based nanoencapsulation stands out for its efficiency, ease of production, and versatility in encapsulating substances, whether hydrophilic or lipophilic. This work aimed at encapsulating pequi oil in liposomes and freeze-dried liposomes to enhance its stability and functional benefits, such as skin hydration and anti-aging effects, for use in innovative cosmetic formulations. Pequi oil—extracted from the Caryocar brasiliense fruit pulp, a plant species from Brazilian plant biodiversity—is rich in secondary metabolites and fatty acids. Liposomes and dried liposomes offer controlled production processes and seamless integration into cosmetic formulations. The physicochemical analysis of the developed liposomes confirmed that the formulations are homogeneous and electrokinetically stable, as evidenced by consistent particle size distribution and zeta potential values, respectively. The gel-type formulations loaded with the dried liposomes exhibit enhanced skin hydration, improved barrier function, and refined microrelief, indicating improvements in skin conditions. These results highlight the potential of dried liposomes containing pequi oil for the development of innovative cosmeceutical products. This research contributes to the valorization of Brazilian biodiversity by presenting an innovative approach to leveraging the dermatological benefits of pequi oil in cosmetic applications.
Full article
(This article belongs to the Special Issue Spray Drying and Encapsulation of Pharmaceuticals and Phytopharmaceuticals)
►▼
Show Figures

Figure 1
Open AccessArticle
Mass Production of Rg1-Loaded Small Extracellular Vesicles Using a 3D Bioreactor System for Enhanced Cardioprotective Efficacy of Doxorubicin-Induced Cardiotoxicity
by
Yunfeng Di, Shuang Zhao, Huilan Fan, Wei Li, Guangjian Jiang, Yong Wang, Chun Li, Wei Wang and Jingyu Wang
Pharmaceutics 2024, 16(5), 593; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050593 (registering DOI) - 26 Apr 2024
Abstract
Background: Small extracellular vesicles (sEVs) obtained from human umbilical cord mesenchymal stromal cells (MSCs) have shown cardioprotective efficacy in doxorubicin-induced cardiotoxicity (DIC). However, their clinical application is limited due to the low yield and high consumption. This study aims to achieve large-scale production
[...] Read more.
Background: Small extracellular vesicles (sEVs) obtained from human umbilical cord mesenchymal stromal cells (MSCs) have shown cardioprotective efficacy in doxorubicin-induced cardiotoxicity (DIC). However, their clinical application is limited due to the low yield and high consumption. This study aims to achieve large-scale production of sEVs using a three-dimensional (3D) bioreactor system. In addition, sEVs were developed to deliver Ginsenoside Rg1 (Rg1), a compound derived from traditional Chinese medicine, Ginseng, that has cardioprotective properties but limited bioavailability, to enhance the treatment of DIC. Methods: The 3D bioreactor system with spinner flasks was used to expand human umbilical cord MSCs and collect MSC-conditioned medium. Subsequently, sEVs were isolated from the conditioned medium using differential ultra-centrifugation (dUC). The sEVs were loaded with Ginsenoside Rg1 by electroporation and evaluated for cardioprotective efficacy using Cell Counting Kit-8 (CCK-8) analysis, Annexin V/PI staining and live cell count of H9c2 cells under DIC. Results: Using the 3D bioreactor system with spinner flasks, the expansion of MSCs reached ~600 million, and the production of sEVs was up to 2.2 × 1012 particles in five days with significantly reduced bench work compared to traditional 2D flasks. With the optimized protocol, the Ginsenoside Rg1 loading efficiency of sEVs by electroporation was ~21%, higher than sonication or co-incubation. Moreover, Rg1-loaded sEVs had attenuated DOX-induced cardiotoxicity with reduced apoptosis compared to free Ginsenoside Rg1 or sEVs. Conclusions: The 3D culture system scaled up the production of sEVs, which facilitated the Rg1 delivery and attenuated cardiomyocyte apoptosis, suggesting a potential treatment of DOX-induced cardiotoxicity.
Full article
(This article belongs to the Special Issue Advances in Exosomes in Drug Delivery Systems)
Open AccessArticle
Distinguishing Molecular Properties of OAT, OATP, and MRP Drug Substrates by Machine Learning
by
Anisha K. Nigam, Jeremiah D. Momper, Anupam Anand Ojha and Sanjay K. Nigam
Pharmaceutics 2024, 16(5), 592; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050592 (registering DOI) - 26 Apr 2024
Abstract
The movement of organic anionic drugs across cell membranes is partly governed by interactions with SLC and ABC transporters in the intestine, liver, kidney, blood–brain barrier, placenta, breast, and other tissues. Major transporters involved include organic anion transporters (OATs, SLC22 family), organic anion
[...] Read more.
The movement of organic anionic drugs across cell membranes is partly governed by interactions with SLC and ABC transporters in the intestine, liver, kidney, blood–brain barrier, placenta, breast, and other tissues. Major transporters involved include organic anion transporters (OATs, SLC22 family), organic anion transporting polypeptides (OATPs, SLCO family), and multidrug resistance proteins (MRPs, ABCC family). However, the sets of molecular properties of drugs that are necessary for interactions with OATs (OAT1, OAT3) vs. OATPs (OATP1B1, OATP1B3) vs. MRPs (MRP2, MRP4) are not well-understood. Defining these molecular properties is necessary for a better understanding of drug and metabolite handling across the gut–liver–kidney axis, gut–brain axis, and other multi-organ axes. It is also useful for tissue targeting of small molecule drugs and predicting drug–drug interactions and drug–metabolite interactions. Here, we curated a database of drugs shown to interact with these transporters in vitro and used chemoinformatic approaches to describe their molecular properties. We then sought to define sets of molecular properties that distinguish drugs interacting with OATs, OATPs, and MRPs in binary classifications using machine learning and artificial intelligence approaches. We identified sets of key molecular properties (e.g., rotatable bond count, lipophilicity, number of ringed structures) for classifying OATs vs. MRPs and OATs vs. OATPs. However, sets of molecular properties differentiating OATP vs. MRP substrates were less evident, as drugs interacting with MRP2 and MRP4 do not form a tight group owing to differing hydrophobicity and molecular complexity for interactions with the two transporters. If the results also hold for endogenous metabolites, they may deepen our knowledge of organ crosstalk, as described in the Remote Sensing and Signaling Theory. The results also provide a molecular basis for understanding how small organic molecules differentially interact with OATs, OATPs, and MRPs.
Full article
Open AccessArticle
In Vitro Stability and Pharmacokinetic Study of Pedunculoside and Its Beta-CD Polymer Inclusion Complex
by
Liang Wu, Danfeng Li, Peijing Wang, Linling Dong, Wang Zhang, Jianjun Xu and Xiaoliang Jin
Pharmaceutics 2024, 16(5), 591; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050591 - 26 Apr 2024
Abstract
Pedunculoside, a triterpene saponin derived from various Ilex species, holds potential as a treatment for cardiovascular diseases. However, its clinical application is hindered by poor bioavailability, rapid elimination, and extensive intestinal metabolism to rotundic acid. To address these issues, a water-soluble inclusion complex
[...] Read more.
Pedunculoside, a triterpene saponin derived from various Ilex species, holds potential as a treatment for cardiovascular diseases. However, its clinical application is hindered by poor bioavailability, rapid elimination, and extensive intestinal metabolism to rotundic acid. To address these issues, a water-soluble inclusion complex of pedunculoside, namely, the beta-CD polymer inclusion complex of pedunculoside (pedunculoside–βCDP), was prepared in this study, and a comparative in vitro stability and pharmacokinetic behavior study was performed between pedunculoside and pedunculoside–βCDP. Both pedunculoside and pedunculoside–βCDP exhibited the highest stability in simulated gastric fluid and simulated intestinal fluid but were readily metabolized when co-incubated with Bifidobacterium adolescentis and Bifidobacterium breve. An LC-MS/MS analytical method for the simultaneous determination of pedunculoside and rotundic acid in rat plasma was successfully established, validated, and applied to investigate the pharmacokinetic behavior after rats were intravenously administered with pedunculoside or pedunculoside–βCDP. The results indicated that pedunculoside–βCDP could significantly improve the pharmacokinetic profile of pedunculoside by increasing plasma exposure, retarding elimination, and reducing intestinal metabolism. This study enhances our understanding of pedunculoside–βCDP’s metabolic fate and pharmacokinetic properties and potentially advances its further research, development, and clinical application.
Full article
(This article belongs to the Special Issue Pharmacokinetics, Pharmacodynamics and Drug Interactions)
Open AccessArticle
Co-Delivery of an Innovative Organoselenium Compound and Paclitaxel by pH-Responsive PCL Nanoparticles to Synergistically Overcome Multidrug Resistance in Cancer
by
Daniela Mathes, Letícia Bueno Macedo, Taís Baldissera Pieta, Bianca Costa Maia, Oscar Endrigo Dorneles Rodrigues, Julliano Guerin Leal, Marcelo Wendt, Clarice Madalena Bueno Rolim, Montserrat Mitjans and Daniele Rubert Nogueira-Librelotto
Pharmaceutics 2024, 16(5), 590; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050590 - 26 Apr 2024
Abstract
In this study, we designed the association of the organoselenium compound 5′-Seleno-(phenyl)-3′-(ferulic-amido)-thymidine (AFAT-Se), a promising innovative nucleoside analogue, with the antitumor drug paclitaxel, in poly(ε-caprolactone) (PCL)-based nanoparticles (NPs). The nanoprecipitation method was used, adding the lysine-based surfactant, 77KS, as a pH-responsive adjuvant. The
[...] Read more.
In this study, we designed the association of the organoselenium compound 5′-Seleno-(phenyl)-3′-(ferulic-amido)-thymidine (AFAT-Se), a promising innovative nucleoside analogue, with the antitumor drug paclitaxel, in poly(ε-caprolactone) (PCL)-based nanoparticles (NPs). The nanoprecipitation method was used, adding the lysine-based surfactant, 77KS, as a pH-responsive adjuvant. The physicochemical properties presented by the proposed NPs were consistent with expectations. The co-nanoencapsulation of the bioactive compounds maintained the antioxidant activity of the association and evidenced greater antiproliferative activity in the resistant/MDR tumor cell line NCI/ADR-RES, both in the monolayer/two-dimensional (2D) and in the spheroid/three-dimensional (3D) assays. Hemocompatibility studies indicated the safety of the nanoformulation, corroborating the ability to spare non-tumor 3T3 cells and human mononuclear cells of peripheral blood (PBMCs) from cytotoxic effects, indicating its selectivity for the cancerous cells. Furthermore, the synergistic antiproliferative effect was found for both the association of free compounds and the co-encapsulated formulation. These findings highlight the antitumor potential of combining these bioactives, and the proposed nanoformulation as a potentially safe and effective strategy to overcome multidrug resistance in cancer therapy.
Full article
(This article belongs to the Section Nanomedicine and Nanotechnology)
►▼
Show Figures
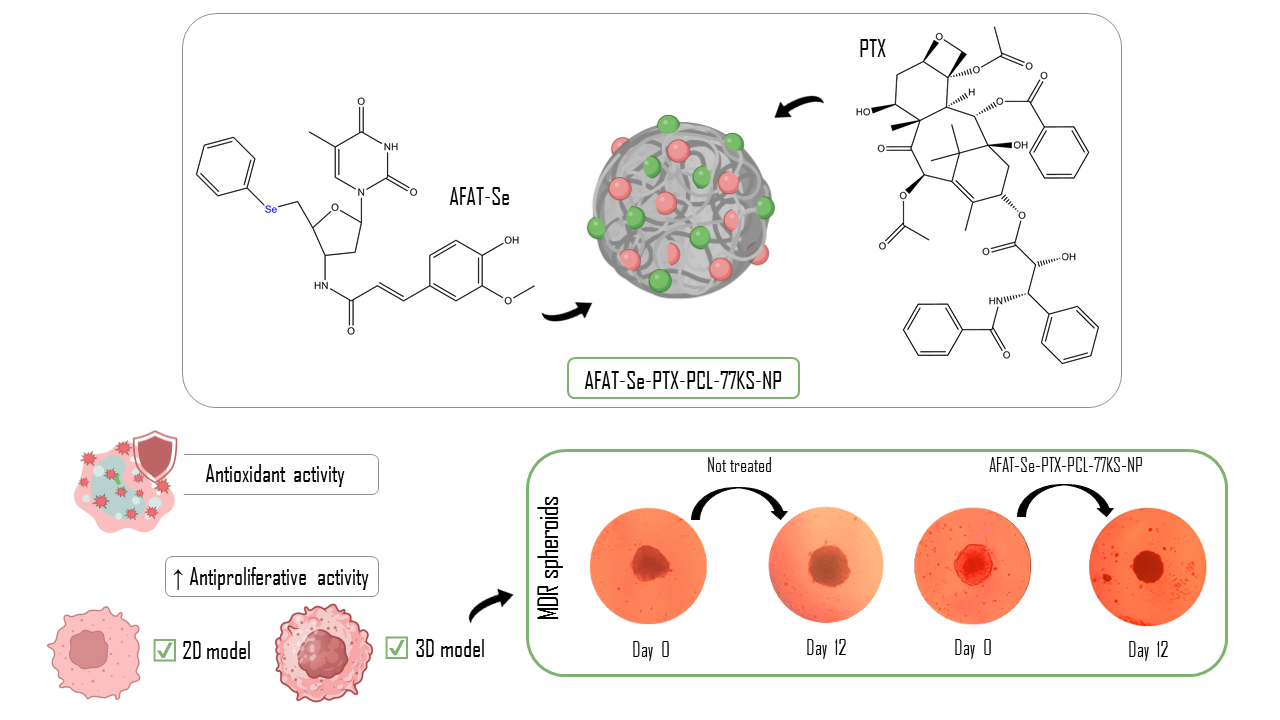
Graphical abstract
Open AccessArticle
QbD Approach-Based Preparation and Optimization of Hydrophobic Ion-Pairing Complex of Lysozyme with Sodium Dodecyl Sulphate to Enhance Stability in Lipid-Based Carriers
by
Alharith A. A. Hassan, Tamás Sovány, Krisztián Pamlényi, Martin Deák, Viktória Hornok, Edit Csapó, Géza Regdon, Jr., Ildikó Csóka and Katalin Kristó
Pharmaceutics 2024, 16(5), 589; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050589 - 26 Apr 2024
Abstract
Hydrophobic ion pairing (HIP) complexation was found to be an efficient approach in modulating the release and enhancing the stability and encapsulation of hydrophilic macromolecules such as proteins in hydrophobic nano/microcarriers. The present work strives to develop and optimize the preparation of the
[...] Read more.
Hydrophobic ion pairing (HIP) complexation was found to be an efficient approach in modulating the release and enhancing the stability and encapsulation of hydrophilic macromolecules such as proteins in hydrophobic nano/microcarriers. The present work strives to develop and optimize the preparation of the HIP complex of the antimicrobial enzyme lysozyme (LYZ) with the ion-pairing agent (IPA) sodium dodecyl sulphate (SDS) relying on the quality-by-design (QbD) approach. The quality target product profile (QTPP) includes the achievement of maximal lipophilicity in a reversible manner to enable the maintenance of biological activity. The related critical quality attributes (CQAs) were defined as complexation efficacy, complex stability, enzyme recovery and activity. Three risk assessment (RA) tools were used to identify and rank the critical process parameters (CPPs) and critical material attributes (CMAs). From this assessment, the pH of the medium, LYZ:SDS molar ratio and drying conditions were determined as high-risk factors that need to be investigated. To the best of our knowledge, for the first time, electrostatic titration was used as a smart approach to determine the optimum molar ratio at different pH values. Based on the predefined CQAs, pH 8 with an LYZ/SDS molar ratio of 1:8 was found to be the optimal condition for complexation efficiency and recovery (%) of a biologically active enzyme. A cost-effective drying process based on a ventilated oven was developed, which resulted in complex qualities comparable to those obtained by the commonly used freeze-drying method. In a nutshell, the optimum conditions for the preparation of the LYZ/SDS HIP complex were efficiently facilitated by the rational application of QbD principles and the utilization of efficient electrostatic titration and ventilated oven-drying methods.
Full article
(This article belongs to the Special Issue Advances in Delivering Protein and Peptide Therapeutics, 2nd Edition)
Open AccessReview
Plant-Derived Vesicle-like Nanoparticles: The Next-Generation Drug Delivery Nanoplatforms
by
Xiaoxia Wang, Congling Xin, Yu Zhou and Tao Sun
Pharmaceutics 2024, 16(5), 588; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050588 - 26 Apr 2024
Abstract
A wide variety of natural bioactive compounds derived from plants have demonstrated significant clinical relevance in the treatment of various diseases such as cancer, chronic disease, and inflammation. An increasing number of studies have surfaced that give credence to the potential of plant-derived
[...] Read more.
A wide variety of natural bioactive compounds derived from plants have demonstrated significant clinical relevance in the treatment of various diseases such as cancer, chronic disease, and inflammation. An increasing number of studies have surfaced that give credence to the potential of plant-derived vesicle-like nanoparticles (PDVLNs) as compelling candidates for a drug delivery system (DDS). PDVLNs are cost-effective production, non-toxicity and non-immunogenicity and fascinating bi-ocompatibility. In this review, we attempt to comprehensively review and consolidate the position of PDVLNs as next-generation drug delivery nanoplatforms. We aim to give a quick glance to readers of the current developments of PDVLNs, including their biogenesis, characteristic features, composition, administration routes, advantages, and application. Further, we discuss the advantages and limitations of PDVLNs. We expect that the role of PDVLNs in drug delivery will be significantly enhanced, thus positioning them as the next generation of therapeutic modalities in the foreseeable future.
Full article
(This article belongs to the Section Drug Delivery and Controlled Release)
►▼
Show Figures
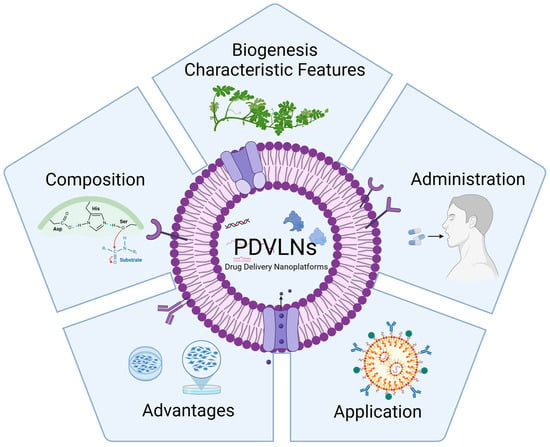
Figure 1
Open AccessArticle
A Novel Intrauterine Device for the Spatio-Temporal Release of Norethindrone Acetate as a Counter-Estrogenic Intervention in the Genitourinary Syndrome of Menopause
by
Ahmed Abdelgader, Mershen Govender, Pradeep Kumar and Yahya E. Choonara
Pharmaceutics 2024, 16(5), 587; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050587 - 26 Apr 2024
Abstract
The genitourinary syndrome of menopause (GSM) is a widely occurring condition affecting millions of women worldwide. The current treatment of GSM involves the use of orally or vaginally administered estrogens, often with the risk of endometrial hyperplasia. The utilization of progestogens offers a
[...] Read more.
The genitourinary syndrome of menopause (GSM) is a widely occurring condition affecting millions of women worldwide. The current treatment of GSM involves the use of orally or vaginally administered estrogens, often with the risk of endometrial hyperplasia. The utilization of progestogens offers a means to counteract the effects of estrogen on the endometrial tissue, decreasing unwanted side effects and improving therapeutic outcomes. In this study, a norethindrone acetate (NETA)-loaded, hollow, cylindrical, and sustained release platform has been designed, fabricated, and optimized for implantation in the uterine cavity as a counter-estrogenic intervention in the treatment of GSM. The developed system, which comprises ethyl cellulose (EC) and polycaprolactone (PCL), has been statistically optimized using a two-factor, two-level factorial design, with the mechanical properties, degradation, swelling, and in vitro drug release of NETA from the device evaluated. The morphological characteristics of the platform were further investigated through scanning electron microscopy in addition to cytocompatibility studies using NIH/3T3 cells. Results from the statistical design highlighted the platform with the highest NETA load and the EC-to-PCL ratio that exhibited favorable release and weight loss profiles. The drug release data for the optimal formulation were best fitted with the Peppas–Sahlin model, implicating both diffusion and polymer relaxation in the release mechanism, with cell viability results noting that the prepared platform demonstrated favorable cytocompatibility. The significant findings of this study firmly establish the developed platform as a promising candidate for the sustained release of NETA within the uterine cavity. This functionality serves as a counter-estrogenic intervention in the treatment of GSM, with the platform holding potential for further advanced biomedical applications.
Full article
(This article belongs to the Special Issue Drug Delivery in the Reproductive Systems)
►▼
Show Figures
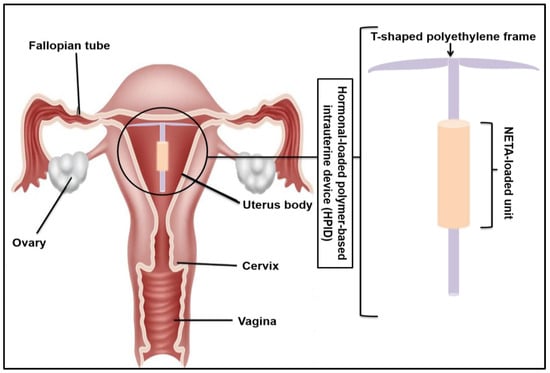
Figure 1
Open AccessArticle
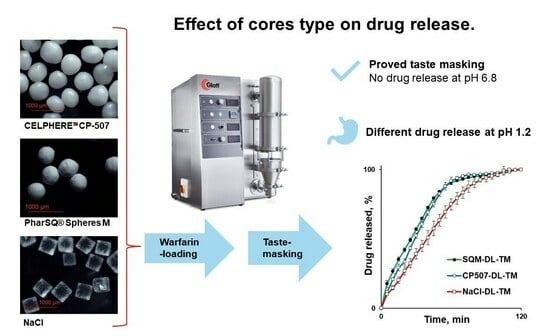
Taste-Masked Pellets of Warfarin Sodium: Formulation towards the Dose Personalisation
by
Lakija Kovalenko, Kirils Kukuls, Marta Berga and Valentyn Mohylyuk
Pharmaceutics 2024, 16(5), 586; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050586 - 26 Apr 2024
Abstract
The bitter drug, warfarin, has a narrow therapeutic index (NTI) and is used in paediatrics and geriatrics. The aim of this feasibility study was to formulate the taste-masked warfarin-containing pellets to be applicable for dose personalisation and to improve patient compliance, as well
[...] Read more.
The bitter drug, warfarin, has a narrow therapeutic index (NTI) and is used in paediatrics and geriatrics. The aim of this feasibility study was to formulate the taste-masked warfarin-containing pellets to be applicable for dose personalisation and to improve patient compliance, as well as to investigate the effect of the core type (PharSQ® Spheres M, CELPHERE™ CP-507, and NaCl) on the warfarin release from the Kollicoat® Smartseal taste-masking-coated pellets. The cores were successfully drug-loaded and coated in a fluid-bed coater with a Wurster insert. An increase in particle size and particle size distribution was observed by optical microscopy. In saliva-simulated pH, at the Kollicoat® Smartseal level of 2 mg/cm2, none of the pellets demonstrated drug release, confirming their efficient taste-masking. However, in a stomach-simulated pH, a faster drug release was observed from PharSQ® Spheres M- and CELPHERE™ CP-507-coated pellets in comparison with NaCl cores. Additional experiments allowed us to explain the slower drug release from NaCl-containing pellets because of the salting-out effect. Despite the successful taste masking, the drug release from pellets was relatively slow (not more than 91% per 60 min), allowing for further formulation improvements.
Full article
(This article belongs to the Special Issue Dosage Form Design for Oral Administration)
►▼
Show Figures
Graphical abstract
Open AccessEditorial
Editorial for Special Issue ‘Engineering and Characterisation of Novel Nanomedicine Formulations’
by
Raquel Fernández-García, Francisco Bolás-Fernández and Ana Isabel Fraguas-Sánchez
Pharmaceutics 2024, 16(5), 585; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050585 - 26 Apr 2024
Abstract
Nanomedicine is the application of nanotechnology to achieve innovations in healthcare and involves the engineering of systems at the nanoscale (particle size < 1000 nm) with the aim of improving drug delivery [...]
Full article
(This article belongs to the Special Issue Engineering and Characterisation of Novel Nanomedicine Formulations)
Open AccessArticle
Point Prevalence Survey of Acute Hospital Patients with Difficulty Swallowing Solid Oral Dose Forms
by
Anne Harnett, Stephen Byrne, Jennifer O’Connor, Eimear Burke, Laura South, Declan Lyons and Laura J. Sahm
Pharmaceutics 2024, 16(5), 584; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050584 - 25 Apr 2024
Abstract
The safe administration of solid oral dose forms in hospital inpatients with swallowing difficulties is challenging. The aim of this study was to establish the prevalence of difficulties in swallowing solid oral dose forms in acute hospital inpatients. A point prevalence study was
[...] Read more.
The safe administration of solid oral dose forms in hospital inpatients with swallowing difficulties is challenging. The aim of this study was to establish the prevalence of difficulties in swallowing solid oral dose forms in acute hospital inpatients. A point prevalence study was completed at three time points. The following data were collected: the prevalence of swallowing difficulties, methods used to modify solid oral dose forms to facilitate administration, the appropriateness of the modification, and patient co-morbidities. The prevalence of acute hospital inpatients with swallowing difficulties was an average of 15.4% with a 95% CI [13.4, 17.6] across the three studies. On average, 9.6% of patients with swallowing difficulties had no enteral feeding tube in situ, with 6.0% of these patients receiving at least one modified medicine. The most common method of solid oral dose form modification was crushing, with an administration error rate of approximately 14.4%. The most common co-morbid condition in these patients was hypertension, with dysphagia appearing on the problem list of two (5.5%) acute hospital inpatients with swallowing difficulties. Inappropriate modifications to solid oral dose forms to facilitate administration can result in patient harm. A proactive approach, such as the use of a screening tool to identify acute hospital inpatients with swallowing difficulties, is required, to mitigate the risk of inappropriate modifications to medicines to overcome swallowing difficulties.
Full article
(This article belongs to the Special Issue Emerging Strategies in Drug Development and Clinical Care in the Era of Personalized and Precision Medicine)
Open AccessReview
Therapeutic Effects of Essential Oils and Their Bioactive Compounds on Prostate Cancer Treatment
by
Leticia Santos Pimentel, Luciana Machado Bastos, Luiz Ricardo Goulart and Lígia Nunes de Morais Ribeiro
Pharmaceutics 2024, 16(5), 583; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050583 - 24 Apr 2024
Abstract
Since prostate cancer (PCa) relies on limited therapies, more effective alternatives are required. Essential oils (EOs) and their bioactive compounds are natural products that have many properties including anticancer activity. This review covers studies published between 2000 and 2023 and discusses the anti-prostate
[...] Read more.
Since prostate cancer (PCa) relies on limited therapies, more effective alternatives are required. Essential oils (EOs) and their bioactive compounds are natural products that have many properties including anticancer activity. This review covers studies published between 2000 and 2023 and discusses the anti-prostate cancer mechanisms of the EOs from several plant species and their main bioactive compounds. It also provides a critical perspective regarding the challenges to be overcome until they reach the market. EOs from chamomile, cinnamon, Citrus species, turmeric, Cymbopogon species, ginger, lavender, Mentha species, rosemary, Salvia species, thyme and other species have been tested in different PCa cell lines and have shown excellent results, including the inhibition of cell growth and migration, the induction of apoptosis, modulation in the expression of apoptotic and anti-apoptotic genes and the suppression of angiogenesis. The most challenging aspects of EOs, which limit their clinical uses, are their highly lipophilic nature, physicochemical instability, photosensitivity, high volatility and composition variability. The processing of EO-based products in the pharmaceutical field may be an interesting alternative to circumvent EOs' limitations, resulting in several benefits in their further clinical use. Identifying their bioactive compounds, therapeutic effects and chemical structures could open new perspectives for innovative developments in the field. Moreover, this could be helpful in obtaining versatile chemical synthesis routes and/or biotechnological drug production strategies, providing an accurate, safe and sustainable source of these bioactive compounds, while looking at their use as gold-standard therapy in the close future.
Full article
(This article belongs to the Special Issue Natural Products for Anticancer Application)
Open AccessReview
Plant Protease Inhibitors as Emerging Antimicrobial Peptide Agents: A Comprehensive Review
by
Mónica G. Parisi, Brenda Ozón, Sofía M. Vera González, Javier García-Pardo and Walter David Obregón
Pharmaceutics 2024, 16(5), 582; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050582 - 24 Apr 2024
Abstract
Antimicrobial peptides (AMPs) are important mediator molecules of the innate defense mechanisms in a wide range of living organisms, including bacteria, mammals, and plants. Among them, peptide protease inhibitors (PPIs) from plants play a central role in their defense mechanisms by directly attacking
[...] Read more.
Antimicrobial peptides (AMPs) are important mediator molecules of the innate defense mechanisms in a wide range of living organisms, including bacteria, mammals, and plants. Among them, peptide protease inhibitors (PPIs) from plants play a central role in their defense mechanisms by directly attacking pathogens or by modulating the plant’s defense response. The growing prevalence of microbial resistance to currently available antibiotics has intensified the interest concerning these molecules as novel antimicrobial agents. In this scenario, PPIs isolated from a variety of plants have shown potential in inhibiting the growth of pathogenic bacteria, protozoans, and fungal strains, either by interfering with essential biochemical or physiological processes or by altering the permeability of biological membranes of invading organisms. Moreover, these molecules are active inhibitors of a range of proteases, including aspartic, serine, and cysteine types, with some showing particular efficacy as trypsin and chymotrypsin inhibitors. In this review, we provide a comprehensive analysis of the potential of plant-derived PPIs as novel antimicrobial molecules, highlighting their broad-spectrum antimicrobial efficacy, specificity, and minimal toxicity. These natural compounds exhibit diverse mechanisms of action and often multifunctionality, positioning them as promising molecular scaffolds for developing new therapeutic antibacterial agents.
Full article
(This article belongs to the Special Issue Bioactive Molecules from Plants: Discovery and Pharmaceutical Applications, 3rd Edition)
Open AccessArticle
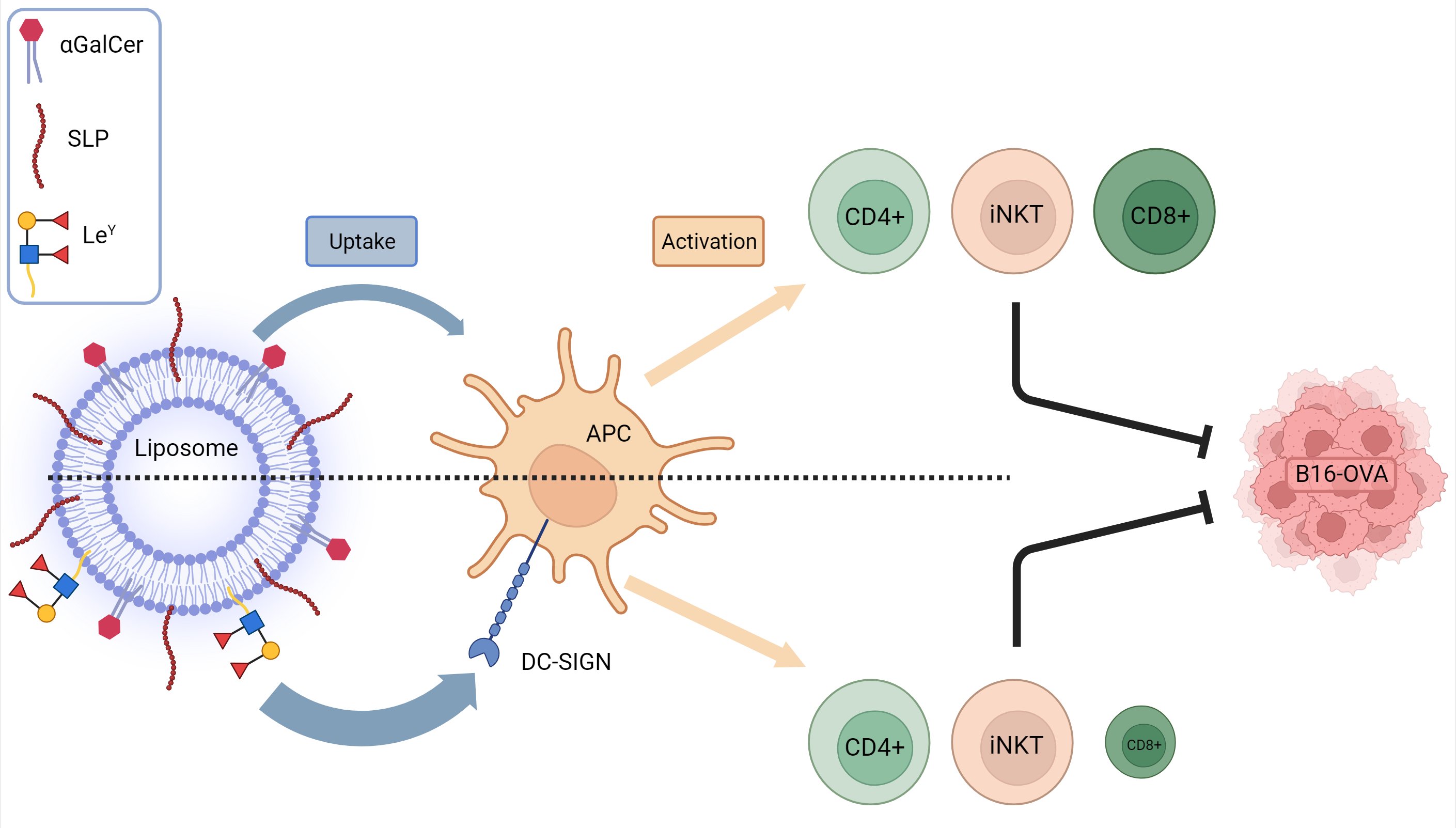
Vaccination with DC-SIGN-Targeting αGC Liposomes Leads to Tumor Control, Irrespective of Suboptimally Activated T-Cells
by
Aram M. de Haas, Dorian A. Stolk, Sjoerd T. T. Schetters, Laura Goossens-Kruijssen, Eelco Keuning, Martino Ambrosini, Louis Boon, Hakan Kalay, Gert Storm, Hans J. van der Vliet, Tanja D. de Gruijl and Yvette van Kooyk
Pharmaceutics 2024, 16(5), 581; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050581 - 24 Apr 2024
Abstract
Cancer vaccines have emerged as a potent strategy to improve cancer immunity, with or without the combination of checkpoint blockade. In our investigation, liposomal formulations containing synthetic long peptides and α-Galactosylceramide, along with a DC-SIGN-targeting ligand, Lewis Y (LeY), were studied
[...] Read more.
Cancer vaccines have emerged as a potent strategy to improve cancer immunity, with or without the combination of checkpoint blockade. In our investigation, liposomal formulations containing synthetic long peptides and α-Galactosylceramide, along with a DC-SIGN-targeting ligand, Lewis Y (LeY), were studied for their anti-tumor potential. The formulated liposomes boosted with anti-CD40 adjuvant demonstrated robust invariant natural killer (iNKT), CD4+, and CD8+ T-cell activation in vivo. The incorporation of LeY facilitated the targeting of antigen-presenting cells expressing DC-SIGN in vitro and in vivo. Surprisingly, mice vaccinated with LeY-modified liposomes exhibited comparable tumor reduction and survival rates to those treated with untargeted counterparts despite a decrease in antigen-specific CD8+ T-cell responses. These results suggest that impaired induction of antigen-specific CD8+ T-cells via DC-SIGN targeting does not compromise anti-tumor potential, hinting at alternative immune activation routes beyond CD8+ T-cell activation.
Full article
(This article belongs to the Special Issue Advanced Liposomes for Drug Delivery, 2nd Edition)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Topical Insulin Eye Drops: Stability and Safety of Two Compounded Formulations for Treating Persistent Corneal Epithelial Defects
by
Marta Vicario-de-la-Torre, Virginia Puebla-García, Lidia Ybañez-García, José Javier López-Cano, Miriam Ana González-Cela-Casamayor, Marco Brugnera, Bárbara Burgos-Blasco, David Díaz-Valle, José Antonio Gegúndez-Fernández, José Manuel Benítez-del-Castillo and Rocío Herrero-Vanrell
Pharmaceutics 2024, 16(5), 580; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050580 - 24 Apr 2024
Abstract
Compounded insulin eye drops were prepared at 1 IU/mL from commercially available subcutaneous insulin by dilution in saline solution or artificial tears. Physicochemical characterization and in vitro tolerance testing in human and conjunctival cells were followed by a 28-day short-term stability study under
[...] Read more.
Compounded insulin eye drops were prepared at 1 IU/mL from commercially available subcutaneous insulin by dilution in saline solution or artificial tears. Physicochemical characterization and in vitro tolerance testing in human and conjunctival cells were followed by a 28-day short-term stability study under various conditions. The formulations were isotonic (280–300 mOsm/L), had a pH close to neutral (7–8), medium surface-tension values (<56 MN/m−1), and low (≈1 mPa·s) and medium (≈5 mPa·s) viscosities (compounded normal saline solution and artificial tear-based preparation, respectively). These values remained stable for 28 days under refrigeration. Microbiological stability was also excellent. Insulin potency remained in the 90–110% range in the compounded formulations containing normal saline solution when stored at 2–8 °C for 28 days, while it decreased in those based on artificial tears. Although both formulations were well tolerated in vitro, the compounded insulin diluted in a normal saline solution exhibited better cell tolerance. Preliminary data in humans showed that insulin in saline solution was an effective and safe treatment for persistent corneal epithelial defects. Compounded insulin eye drops diluted in normal saline solution could, therefore, constitute an emergent therapy for the treatment of persistent corneal epithelial defects.
Full article
(This article belongs to the Special Issue Pharmacy Compounding of Personalized Preparation for Specific Patients: Challenges and Advantages)
Open AccessReview
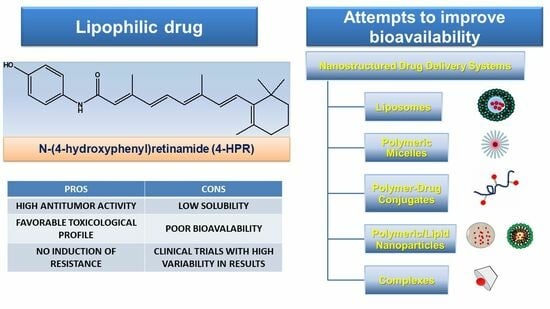
Attempts to Improve Lipophilic Drugs’ Solubility and Bioavailability: A Focus on Fenretinide
by
Silvana Alfei and Guendalina Zuccari
Pharmaceutics 2024, 16(5), 579; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050579 - 24 Apr 2024
Abstract
The development of numerous drugs is often arrested at clinical testing stages, due to their unfavorable biopharmaceutical characteristics. It is the case of fenretinide (4-HPR), a second-generation retinoid, that demonstrated promising in vitro cytotoxic activity against several cancer cell lines. Unfortunately, response rates
[...] Read more.
The development of numerous drugs is often arrested at clinical testing stages, due to their unfavorable biopharmaceutical characteristics. It is the case of fenretinide (4-HPR), a second-generation retinoid, that demonstrated promising in vitro cytotoxic activity against several cancer cell lines. Unfortunately, response rates in early clinical trials with 4-HPR did not confirm the in vitro findings, mainly due to the low bioavailability of the oral capsular formulation that was initially developed. Capsular 4-HPR provided variable and insufficient drug plasma levels attributable to the high hepatic first-pass effect and poor drug water solubility. To improve 4-HPR bioavailability, several approaches have been put forward and tested in preclinical and early-phase clinical trials, demonstrating generally improved plasma levels and minimal systemic toxicities, but also modest antitumor efficacy. The challenge is thus currently still far from being met. To redirect the diminished interest of pharmaceutical companies toward 4-HPR and promote its further clinical development, this manuscript reviewed the attempts made so far by researchers to enhance 4-HPR bioavailability. A comparison of the available data was performed, and future directions were proposed.
Full article
(This article belongs to the Special Issue Pharmaceutics and Drug Delivery in Italy, 2nd Edition)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Characterization of Oil-in-Water Emulsions Prepared with Triblock Copolymer Poloxamer 407 and Low-Molecular-Mass Surfactant Mixtures as Carriers of Grape Pomace Waste Polyphenols
by
Veljko S. Krstonošić, Darija B. Sazdanić, Dejan M. Ćirin, Ivana R. Nikolić, Miroslav S. Hadnađev and Milica T. Atanacković Krstonošić
Pharmaceutics 2024, 16(5), 578; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050578 - 24 Apr 2024
Abstract
Background: Natural antioxidants, such as grape pomace polyphenols, can be extracted by a surfactant-based green technology and incorporated into various emulsions. Therefore, this work aimed to investigate the physical stability and rheological characteristics of oil-in-water emulsions stabilized with poloxamer 407 (P407) and its
[...] Read more.
Background: Natural antioxidants, such as grape pomace polyphenols, can be extracted by a surfactant-based green technology and incorporated into various emulsions. Therefore, this work aimed to investigate the physical stability and rheological characteristics of oil-in-water emulsions stabilized with poloxamer 407 (P407) and its mixtures with the low-molecular-mass surfactants Brij S20 (BS20) and Tween 60 (T60). Also, the influence of polyphenolic grape pomace extracts on the physical stability and rheological characteristics of the emulsions was examined. Methods: Grape pomace polyphenols were extracted by aqueous solutions of P407 and BS20/P407 and T60/P407 mixtures. Two different types of oil-in-water emulsions were examined: emulsions prepared with pure surfactants and emulsions prepared with surfactant-based polyphenol extracts of grape pomace. Both types contained 20% sunflower oil. Characterization of the emulsions comprised droplet size evaluation, rheology characteristics and creaming stability. Results: All the emulsions showed shear-thinning flow, while the rheological characteristics and creaming instability depended on the proportion of P407 in the emulsifier mixtures. Incorporation of grape pomace extracts had no effect on the investigated properties of the emulsions. Conclusion: The presence of extracted polyphenols in emulsifier mixtures had no significant effects on the emulsions’ physico-chemical characteristics and stability. Therefore, the investigated emulsions can be considered suitable carriers for polyphenol-rich extracts.
Full article
(This article belongs to the Special Issue Emulsions, Polymers and Micelles for Drug Delivery Applications)
►▼
Show Figures
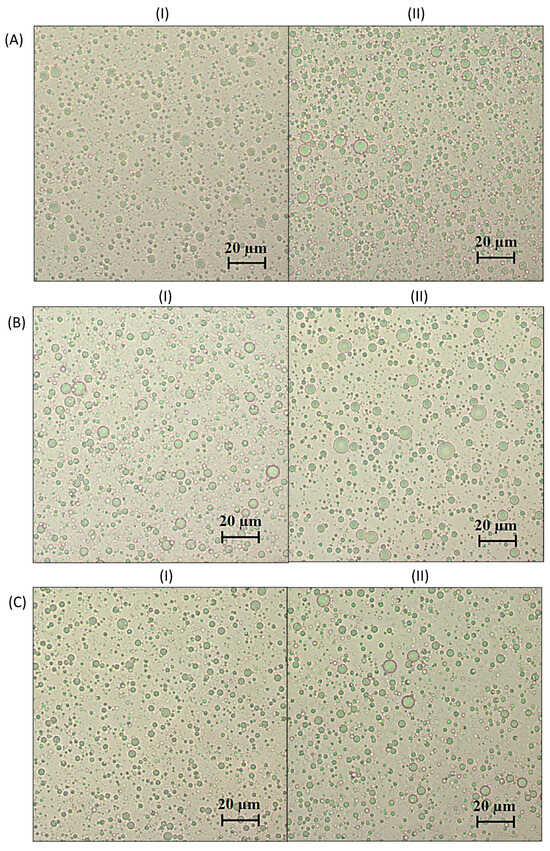
Figure 1
Open AccessReview
Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention
by
Rosa Direito, Sandra Maria Barbalho, Bruno Sepodes and Maria Eduardo Figueira
Pharmaceutics 2024, 16(5), 577; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050577 - 24 Apr 2024
Abstract
There is a growing trend among consumers to seek out natural foods and products with natural ingredients. This shift in consumer preferences had a direct impact on both food and pharmaceutical industries, leading to a focus of scientific research and commercial efforts to
[...] Read more.
There is a growing trend among consumers to seek out natural foods and products with natural ingredients. This shift in consumer preferences had a direct impact on both food and pharmaceutical industries, leading to a focus of scientific research and commercial efforts to meet these new demands. The aim of this work is to review recent available scientific data on foods of interest, such as the artichoke, gooseberry, and polygonoideae plants, as well as olive oil and red raspberries. Interestingly, the urgency of solutions to the climate change emergency has brought new attention to by-products of grapevine bunch stem and cane, which have been found to contain bioactive compounds with potential health benefits. There is a pressing need for a faster process of translating scientific knowledge from the laboratory to real-world applications, especially in the face of the increasing societal burden associated with non-communicable diseases (NCDs), environmental crises, the post-pandemic world, and ongoing violent conflicts around the world.
Full article
(This article belongs to the Special Issue Nanotechnology and Natural Products: Plant Bioactive Compounds for Drug Delivery (Volume II))
►▼
Show Figures
Figure 1
Open AccessArticle
Pharmaceutical Compounding in Veterinary Medicine: Suspension of Itraconazole
by
Gema J. Cabañero-Resta, Bárbara Sánchez-Dengra, Alejandro Ruiz-Picazo, Marival Bermejo, Virginia Merino, Isabel Gonzalez-Alvarez and Marta Gonzalez-Alvarez
Pharmaceutics 2024, 16(5), 576; https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050576 - 24 Apr 2024
Abstract
Itraconazole is a drug used in veterinary medicine for the treatment of different varieties of dermatophytosis at doses between 3–5 mg/kg/day in cats. Nevertheless, in Spain, it is only available in the market as a 52 mL suspension at 10 mg/mL. The lack
[...] Read more.
Itraconazole is a drug used in veterinary medicine for the treatment of different varieties of dermatophytosis at doses between 3–5 mg/kg/day in cats. Nevertheless, in Spain, it is only available in the market as a 52 mL suspension at 10 mg/mL. The lack of alternative formulations, which provide sufficient formulation to cover the treatment of large animals or allow the treatment of a group of them, can be overcome with compounding. For this purpose, it has to be considered that itraconazole is a weak base, class II compound, according to the Biopharmaceutics Classification System, that can precipitate when reaching the duodenum. The aim of this work is to develop alternative oral formulations of itraconazole for the treatment of dermatophytosis. Several oral compounds of itraconazole were prepared and compared, in terms of dissolution rate, permeability, and stability, in order to provide alternatives to the medicine commercialized. The most promising formulation contained hydroxypropyl methylcellulose and β-cyclodextrin. This combination of excipients was capable of dissolving the same concentration as the reference product and delaying the precipitation of itraconazole upon leaving the stomach. Moreover, the intestinal permeability of itraconazole was increased more than two-fold.
Full article
(This article belongs to the Special Issue Dose Optimization of Traditional and New Drug Formulations in Veterinary and Human Medicine)
►▼
Show Figures
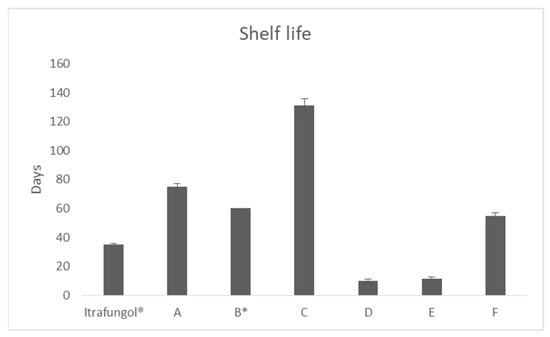
Figure 1

Journal Menu
► ▼ Journal Menu-
- Pharmaceutics Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Pharmaceuticals, Pharmaceutics, Antioxidants, Nanomaterials, JFB, Cosmetics
New Challenges in the Cosmetics and Medical Device Industry
Topic Editors: Ana Catarina Silva, Hugo Almeida, Ana BarrosDeadline: 30 April 2024
Topic in
Bioengineering, Biomolecules, Cancers, Diseases, Nanomaterials, Pharmaceutics
Dynamic Nano-Biomaterials in Tissue Regeneration and Cancer Therapies
Topic Editors: Ramar Thangam, Heemin Kang, Bibin G. Anand, Ramachandran Vijayan, Venugopal KrishnanDeadline: 15 May 2024
Topic in
Cancers, Cells, Diseases, Nanomaterials, Pharmaceutics
Findings, Insights and Perspectives on Central Nervous System Tumors
Topic Editors: João Basso, Carla Vitorino, Rui Vitorino, Ana FortunaDeadline: 31 May 2024
Topic in
Cancers, Cells, JCM, Radiation, Pharmaceutics, Applied Sciences, Nanomaterials, Current Oncology
Innovative Radiation Therapies
Topic Editors: Gérard Baldacchino, Eric Deutsch, Marie Dutreix, Sandrine Lacombe, Erika Porcel, Charlotte Robert, Emmanuelle Bourneuf, João Santos Sousa, Aurélien de la LandeDeadline: 30 June 2024

Conferences
Special Issues
Special Issue in
Pharmaceutics
Advanced Nanomaterials for Drug Delivery
Guest Editors: Roxana Cristina Popescu, Diana SavuDeadline: 30 April 2024
Special Issue in
Pharmaceutics
New Properties of Supramolecular Complexes and Drug Nanoparticles
Guest Editors: Elena Uspenskaya, Anton SyroeshkinDeadline: 20 May 2024
Special Issue in
Pharmaceutics
Novel Therapeutic Strategies for Glioblastoma
Guest Editors: Milica Pešić, Ana Podolski-Renić, Jelena DinićDeadline: 31 May 2024
Special Issue in
Pharmaceutics
Supramolecular Systems for Gene and Drug Delivery, 2nd Edition
Guest Editors: Francisco José Ostos, José Antonio Lebrón, Pilar López-CornejoDeadline: 10 June 2024
Topical Collections
Topical Collection in
Pharmaceutics
Feature Papers in Pharmaceutical Technology
Collection Editor: Thierry Vandamme
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Korea
Collection Editors: Hyo-Kyung Han, Beom-Jin Lee
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Estonia
Collection Editors: Karin Kogermann, Jana Lass
Topical Collection in
Pharmaceutics
Women in Pharmaceutics
Collection Editors: Donatella Paolino, Cinzia Anna Ventura