Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
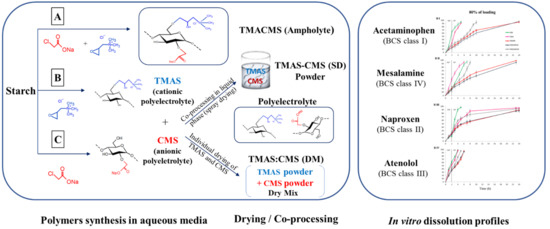
2.2.1. Synthesis of Starch Derivatives
2.2.2. Degree of Substitution (DS)
2.2.3. Fourier Transform Infrared (FT-IR)
2.2.4. X-ray Diffraction
2.2.5. Zeta Potential (ζ)
2.2.6. pH Determination
2.2.7. Scanning Electron Microscopy (SEM)
2.2.8. Thermogravimetric Analyses (TGA)
2.2.9. Micromeritic Analyses
2.2.10. Preparation of Tablets
2.2.11. Determination of Swelling, Fluid Uptake and Erosion
2.2.12. In Vitro Dissolution Tests
3. Results
3.1. Structural Properties
3.2. Fourier Transform Infrared (FT-IR)
3.3. X-ray Diffraction Analysis
3.4. Thermogravimetric Analysis (TGA)
3.5. Scanning Electron Microscopy (SEM)
3.6. Micromeritic Analyses
3.7. Fluid Uptake, Swelling and Erosion
3.8. In Vitro Dissolution Assays
4. Discussions
4.1. Fourier Transform Infrared (FT-IR)
4.2. X-ray Diffraction Analysis
4.3. Thermogravimetric Analyses (TGA)
4.4. Scanning Electron Microscopy (SEM)
4.5. Micromeritic Analyses
4.6. Fluid Up-Take, Swelling, and Erosion
4.7. In Vitro Dissolution Assays
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TMAS | trimethylaminestarch |
| CMS | carboxymethylstarch |
| SD | spray drying |
| DM | dry mix |
| TMACMS | trimethylaminecarboxymethylstarch |
| BCS | Biopharmaceutical Classification System |
| API | active pharmaceutical ingredient |
| DS | degree of substitution |
| PEC | polyelectrolyte complexes |
| SMCA | sodium monochloroacetate |
| GTMAC | glycidyltrimethylammonium chloride |
| CI | compressibility index |
| HF | Hausner’s factor |
| USP | United States Pharmacopeia |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| FTIR | Fourier transform infrared spectroscopy |
| DRX | X-ray diffraction |
| TGA | thermogravimetric analysis |
| SEM | scanning electron microscopy |
References
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, W.; He, Z.; Zhang, M.; Kong, F.; Sain, M. Bio-polymer Substrates in Buccal Drug Delivery: Current Status and Future Trend. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ispas-Szabo, P.; de Koninck, P.; Calinescu, C.; Mateescu, M.A. Carboxymethyl Starch Excipients for Drug Chronodelivery. AAPS PharmSciTech 2016, 18, 1673. [Google Scholar] [CrossRef] [PubMed]
- Keraliya, R.A.; Patel, C.; Patel, P.; Keraliya, V.; Soni, T.G.; Patel, R.C.; Patel, M.M. Osmotic drug delivery system as a part of modified release dosage form. ISRN Pharm. 2012, 2012, 528079. [Google Scholar] [CrossRef]
- Missaghi, S.; Patel, P.; Farrell, T.P.; Huatan, H.; Rajabi-Siahboomi, A.R. Investigation of critical core formulation and process parameters for osmotic pump oral drug delivery. AAPS PharmSciTech 2014, 15, 149–160. [Google Scholar] [CrossRef]
- Son, J.S.; Appleford, M.; Ong, J.L.; Wenke, J.C.; Kim, J.M.; Choi, S.H.; Oh, D.S. Porous hydroxyapatite scaffold with three-dimensional localized drug delivery system using biodegradable microspheres. J. Control. Release 2011, 153, 133–140. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, L.; Yuan, F.; Fu, H.; Shan, W. Preparation of sustained release capsules by electrostatic dry powder coating, using traditional dip coating as reference. Int. J. Pharm. 2018, 543, 345–351. [Google Scholar] [CrossRef]
- Maroni, A.; del Curto, M.D.; Zema, L.; Foppoli, A.; Gazzaniga, A. Film coatings for oral colon delivery. Int. J. Pharm. 2013, 457, 372–394. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E. Controlled Drug Delivery: The Role of Self-Assembling Multi-Task Excipients; Woodhead Publishing, Elsevier: Oxford, UK, 2015. [Google Scholar]
- Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, G.; Gu, Z. Recent advances of starch-based excipients used in extended-release tablets: A review. Drug Deliv. 2016, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Minaev, K.M.; Martynova, D.O.; Zakharov, A.S.; Sagitov, R.R.; Ber, A.A.; Ulyanova, O.S. Synthesis of Carboxymethyl Starch for increasing drilling mud quality in drilling oil and gas wells. IOP Conf. Ser. Earth Environ. Sci. 2016, 43, 012071. [Google Scholar] [CrossRef] [Green Version]
- Mateescu, M.A.; Lenaerts, V.; Dumoulin, Y. Use of Cross-Linked Amylose as a Matrix for the Slow Release of Biologically Active Compounds. U.S. Patent 5,456,921, 10 October 1995. [Google Scholar]
- Cartilier, L.; Mateescu, M.A.; Dumoulin, Y.; Lenaerts, V. Cross-Linked Amylose as a Binder/Disintegrant in Tablets. U.S. Patent 5,616,343, 1 April 1997. [Google Scholar]
- Ispas-Szabo, P.; Ravenelle, F.; Hassan, I.; Preda, M.; Mateescu, M.A. Structure–properties relationship in cross-linked high-amylose starch for use in controlled drug release. Carbohydr. Res. 2000, 323, 163–175. [Google Scholar] [CrossRef]
- Calinescu, C.; Mulhbacher, J.; Nadeau, E.; Fairbrother, J.M.; Mateescu, M.A. Carboxymethyl high amylose starch (CM-HAS) as excipient for Escherichia coli oral formulations. Eur. J. Pharm. Biopharm. 2005, 60, 53–60. [Google Scholar] [CrossRef]
- Friciu, M.; Le, T.C.; Ispas-Szabo, P.; Mateescu, M.A. Carboxymethyl starch and lecithin complex as matrix for targeted drug delivery: I. Monolithic mesalamine forms for colon delivery. Eur. J. Pharm. Biopharm. 2013, 85, 521–530. [Google Scholar] [CrossRef]
- Calinescu, C.; Mondovi, B.; Federico, R.; Ispas-Szabo, P.; Mateescu, M.A. Carboxymethyl starch: Chitosan monolithic matrices containing diamine oxidase and catalase for intestinal delivery. Int. J. Pharm. 2012, 428, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blemur, L.; Le, T.C.; Marcocci, L.; Pietrangeli, P.; Mateescu, M.A. Carboxymethyl starch/alginate microspheres containing diamine oxidase for intestinal targeting. Biotechnol. Appl. Biochem. 2016, 63, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, W.; Wang, W.; Wang, W.; Zhao, L.; Li, Y. Sodium carboxymethyl starch-based highly conductive gel electrolyte for quasi-solid-state quantum dot-sensitized solar cells. Res. Chem. Intermed. 2017, 44, 1161–1172. [Google Scholar] [CrossRef]
- Nabais, T.; Leclair, G. High-Amylose Sodium Carboxymethyl Starch Matrices: Development and Characterization of Tramadol Hydrochloride Sustained-Release Tablets for Oral Administration. ISRN Pharm. 2014, 2014, 391–404. [Google Scholar] [CrossRef]
- Massicotte, L.P.; Baille, W.E.; Mateescu, M.A. Carboxylated high amylose starch as pharmaceutical excipients. Structural insights and formulation of pancreatic enzymes. Int. J. Pharm. 2008, 356, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Calinescu, C.; Mateescu, M.A. Carboxymethyl high amylose starch: Chitosan self-stabilized matrix for probiotic colon delivery. Eur. J. Pharm. Biopharm. 2008, 70, 582–589. [Google Scholar] [CrossRef]
- Lemieux, M.; Gosselin, P.; Mateescu, M.A. Influence of drying procedure and of low degree of substitution on the structural and drug release properties of carboxymethyl starch. AAPS PharmSciTech 2010, 11, 775–785. [Google Scholar] [CrossRef]
- Subramanian, S.B.; Francis, A.P.; Devasena, T. Chitosan-starch nanocomposite particles as a drug carrier for the delivery of bis-desmethoxy curcumin analog. Carbohydr. Polym. 2014, 114, 170–178. [Google Scholar] [CrossRef]
- Sakeer, K.; Ispas-Szabo, P.; Benyerbah, N.; Mateescu, M.A. Ampholytic starch excipients for high loaded drug formulations: Mechanistic insights. Int. J. Pharm. 2018, 535, 201–216. [Google Scholar] [CrossRef]
- Peng, H.; Zhong, S.; Lin, Q.; Yao, X.; Liang, Z.; Yang, M.; Yin, G.; Liu, Q.; He, H. Removal of both cationic and anionic contaminants by amphoteric starch. Carbohydr. Polym. 2016, 138, 210–214. [Google Scholar] [CrossRef]
- Lin, Q.; Qian, S.; Li, C.; Pan, H.; Wu, Z.; Liu, G. Synthesis, flocculation and adsorption performance of amphoteric starch. Carbohydr. Polym. 2012, 90, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Shimei, X.; Jingli, W.; Ronglan, W.; Jide, W. Effect of degree of substitution on adsorption behavior of Basic Green 4 by highly crosslinked amphoteric starch with quaternary ammonium and carboxyl groups. Carbohydr. Polym. 2006, 66, 55–59. [Google Scholar] [CrossRef]
- Lekniute, E.; Peciulyte, L.; Klimaviciute, R.; Bendoraitiene, J.; Zemaitaitis, A. Structural characteristics and flocculation properties of amphoteric starch. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 95–102. [Google Scholar] [CrossRef]
- Sakeer, K.; Ispas-Szabo, P.; Mateescu, M.A. Self-Stabilizing Ampholytic Starch Excipients for Sustained Release of Highly Soluble Drugs: The Case Study of Metformin. AAPS PharmSciTech 2017, 18, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Sakeer, K.; Scorza, T.; Romero, H.; Ispas-Szabo, P.; Mateescu, M.A. Starch materials as biocompatible supports and procedure for fast separation of macrophages. Carbohydr. Polym. 2017, 163, 108–117. [Google Scholar] [CrossRef]
- USP. US Pharmacopeia 39, National Formulary 34 USP; United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2015. [Google Scholar]
- Ileleji, K.E.; Zhou, B. The angle of repose of bulk corn stover particles. Powder Technol. 2008, 187, 110–118. [Google Scholar] [CrossRef]
- Lemieux, M. Le Carboxyméthyl Amidon de Faible à haut degré de Substitution: Excipient Multifonctionnel Pour des Formes Pharmaceutiques à Administration Orale, in Biochimie; Université du Québec à Montréal: Montréal, QC, Canada, 2012. [Google Scholar]
- Kasten, G.; Lobo, L.; Dengale, S.; Grohganz, H.; Rades, T.; Lobmann, K. In vitro and in vivo comparison between crystalline and co-amorphous salts of naproxen-arginine. Eur. J. Pharm. Biopharm. 2018, 132, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Li, J.; Jiang, S.; Wang, Y.; Zhang, X.; Ding, J.; Yu, T.; Mao, S. Insights into the mechanisms of chitosan-anionic polymers-based matrix tablets for extended drug release. Int. J. Pharm. 2014, 476, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Assaad, E.; Blemur, L.; Lessard, M.; Mateescu, M.A. Polyelectrolyte complex of carboxymethyl starch and chitosan as protein carrier: Oral administration of ovalbumin. J. Biomater. Sci. 2012, 23, 1713–1728. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, B.; Li, H.; Yang, Y.; Yang, H.; Li, A.; Cheng, R. Amphoteric starch-based flocculants can flocculate different contaminants with even opposite surface charges from water through molecular structure control. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 28–35. [Google Scholar] [CrossRef]
- Shalini, S.; Amaranadh, J.; Mahendra, K.; Vinod, K.S. A green method for the preparation of highly stable organic-inorganic hybrid anion-exchange membranes in aqueous media for electrochemical processes. Polym. Chem. 2010, 1, 1302–1312. [Google Scholar]
- Sekhavat Pour, Z.; Makvandi, P.; Ghaemy, M. Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly(vinyl alcohol). Int. J. Biol. Macromol. 2015, 80, 596–604. [Google Scholar] [CrossRef]
- Hong, L.F.; Cheng, L.H.; Lee, C.Y.; Peh, K.K. Characterisation of Physicochemical Properties of Propionylated Corn Starch and Its Application as Stabiliser. Food Technol. Biotechnol. 2015, 53, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Assaad, E.; Mateescu, M.A. The influence of protonation ratio on properties of carboxymethyl starch excipient at various substitution degrees: Structural insights and drug release kinetics. Int. J. Pharm. 2010, 394, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, V.; Chouinard, F.; Mateescu, M.A.; Ispas-Szabo, P. Cross-Linked High Amylose Starch Resistant to Amylase as a Matrix for the Slow Release of Biologically Active Compounds. U.S. patent 6284273B1, 4 September 2001. [Google Scholar]
- Zhang, B.; Tao, H.; Wei, B.; Jin, Z.; Xu, X.; Tian, Y. Correction: Characterization of different substituted carboxymethyl starch microgels and their interactions with lysozyme. PLoS ONE 2015, 10, 89–95. [Google Scholar]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch-Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch-Stärke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Paramakrishnan, N.; Jha, S.; Kumar, K.J. Effect of carboxymethylation on physicochemical, micromeritics and release characteristics of Kyllinga nemoralis starch. Int. J. Biol. Macromol. 2016, 92, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Assaad, E.; Wang, Y.J.; Zhu, X.X.; Mateescu, M.A. Polyelectrolyte complex of carboxymethyl starch and chitosan as drug carrier for oral administration. Carbohydr. Polym. 2011, 84, 1399–1407. [Google Scholar] [CrossRef]
- Smetanova, L.; Stetinova, V.; Kholova, D.; Kunes, M.; Nobilis, M.; Svoboda, Z.; Kvetina, J. Transintestinal transport mechanisms of 5-aminosalicylic acid (in situ rat intestine perfusion, Caco-2 cells) and Biopharmaceutics Classification System. Gen. Physiol. Biophys. 2013, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]







| Polymers | HF | CI (%) | θ (°) |
|---|---|---|---|
| CMS | 1.3 | 26.3 | 27 |
| TMAS | 1.4 | 30.5 | 31.7 |
| TMACMS | 1.2 | 9.5 | 29.5 |
| TMAS-CMS (SD) | 1.3 | 21 | 38.2 |
| TMAS:CMS (DM) | 1.3 | 28 | 30.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benyerbah, N.; Ispas-Szabo, P.; Sakeer, K.; Chapdelaine, D.; Mateescu, M.A. Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery. Pharmaceutics 2019, 11, 253. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11060253
Benyerbah N, Ispas-Szabo P, Sakeer K, Chapdelaine D, Mateescu MA. Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery. Pharmaceutics. 2019; 11(6):253. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11060253
Chicago/Turabian StyleBenyerbah, Nassim, Pompilia Ispas-Szabo, Khalil Sakeer, Daniel Chapdelaine, and Mircea Alexandru Mateescu. 2019. "Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery" Pharmaceutics 11, no. 6: 253. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11060253