Multi-Modulation of Doxorubicin Resistance in Breast Cancer Cells by Poly(l-histidine)-Based Multifunctional Micelles
Abstract
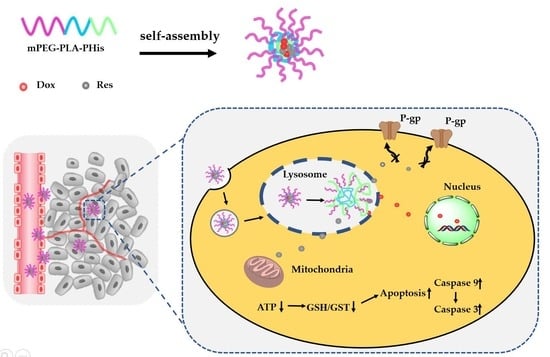
:1. Introduction
2. Materials and Experimental Section
2.1. Materials
2.2. Tested Formulations, Cell Culture and Animals
2.3. Synthesis and Characterizations of pH Sensitive Copolymers
2.4. Preparation and Incorporation Payloads into Micelles
2.5. Characterization of Copolymer Micelles
2.6. In Vitro Release of Dox and Res from Micelles
2.7. In Vitro Cytotoxicity Study
2.8. Intracellular Influx of Dox
2.9. Cellular Uptake and Intracellular Trafficking
2.10. P-gp Expression Determination
2.11. Mitochondrial Membrane Potential Detection
2.12. ATP Contents Assay
2.13. Caspase Activity Assays
2.14. Biodistribution of pHendo-Sensitive Micelles In Vivo
2.15. In Vivo Antitumor Efficacy and Safety Evaluation
2.16. Statistical Analysis
3. Results and Discussion
3.1. Characterizations of mPEG-PLA-PHis by Proton Nuclear Magnetic Resonance (1H-NMR) and GPC
3.2. Characterization of the Micelles
3.3. Enhanced Cytotoxicity Against MCF-7/ADR
3.4. Mechanisms for Reverting MDR
3.4.1. Cellular Uptake and Intracellular Dox Accumulation
3.4.2. Effect on P-gp Expression
3.4.3. Effect on Energy Metabolism Mediated by Mitochondria
3.4.4. Effect on Cell Apoptosis
3.5. In Vivo Biodistribution Studies
3.6. In Vivo Antitumor Efficacy and Safety Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, Y.; Cai, T.; Xia, X.; Zhang, R.; Chiba, P.; Cai, Y. Nanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancer. Drug Deliv. 2016, 23, 3350–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayani, Z.; Firuzi, O.; Bordbar, A.K. Doughnut-shaped bovine serum albumin nanoparticles loaded with doxorubicin for overcoming multidrug-resistant in cancer cells. Int. J. Biol. Macromol. 2018, 107, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavalu, S.; Dermawan, J.K.; Cheriyath, V.; Labhasetwar, V. Highly synergistic effect of sequential treatment with epigenetic and anticancer drugs to overcome drug resistance in breast cancer cells is mediated via activation of p21 gene expression leading to G2/M cycle arrest. Mol. Pharm. 2013, 10, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Kwon, Y.S.; Nam, K.S.; Kim, S. Lapatinib enhances the cytotoxic effects of doxorubicin in MCF-7 tumorspheres by inhibiting the drug efflux function of ABC transporters. Biomed. Pharm. 2015, 72, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Genthe, C.B.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.; Duan, J.; Xiao, Z.; Li, G.; Xu, F. Inhibition of P-glycoprotein and Glutathione S-transferase-pi mediated resistance by fluoxetine in MCF-7/ADM cells. Biomed. Pharmacother. 2013, 67, 757–762. [Google Scholar] [CrossRef]
- Yu, P.; Cheng, X.; Du, Y.; Yang, L.; Huang, L. Significance of MDR-related proteins in the postoperative individualized chemotherapy of gastric cancer. J. Cancer Res. Ther. 2015, 11, 46. [Google Scholar]
- Bal, C.; Baldeyrou, B.; Moz, F.; Lansiaux, A.; Colson, P.; Kraus-Berthier, K.; Léonce, S.; Pierré, A.; Boussard, M.F.; Rousseau, A.; et al. Novel antitumor indenoindole derivatives targeting DNA and topoisomerase II. Biochem. Pharmacol. 2004, 68, 1911–1922. [Google Scholar]
- Kartal-Yandim, M.; Adan-Gokbulut, A.; Baran, Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit. Rev. Biotechnol. 2016, 36, 716–726. [Google Scholar] [CrossRef]
- Sjostedt, N.; Sjöstedt, N.; Holvikari, K.; Tammela, P.; Kidron, H. Inhibition of Breast Cancer Resistance Protein and Multidrug Resistance Associated Protein 2 by Natural Compounds and Their Derivatives. Mol. Pharm. 2017, 14, 135–146. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, L.; Zheng, H.; Gong, X.; Jiang, H.; Chen, J.; Li, Y.; Zheng, H.; Qi, X.; Wang, Y.; et al. Sulfotransferases and Breast Cancer Resistance Protein Determine the Disposition of Calycosin in Vitro and in Vivo. Mol. Pharm. 2017, 14, 2917–2929. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhao, Y.Y.; Shen, J.; Sun, X.; Liu, Y.; Liu, H.; Wang, Y.; Wang, J. Nanoenabled Modulation of Acidic Tumor Microenvironment Reverses Anergy of Infiltrating T Cells and Potentiates Anti-PD-1 Therapy. Nano Lett. 2019, 19, 2774–2783. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Zhang, Q.; Chen, Y.; Zheng, D.; Hao, L.; Duan, C.; Jia, L.; Liu, G.; Liu, Y. Synergistic effect of folate-mediated targeting and verapamil-mediated P-gp inhibition with paclitaxel -polymer micelles to overcome multi-drug resistance. Biomaterials 2011, 32, 9444–9456. [Google Scholar] [CrossRef]
- Hong, W.; Chen, D.; Zhang, X.; Zeng, J.; Hu, H.; Zhao, X.; Qiao, M. Reversing multidrug resistance by intracellular delivery of Pluronic® P85 unimers. Biomaterials 2013, 34, 9602–9614. [Google Scholar] [CrossRef]
- Engblom, P.; Pulkkinen, J.O.; Rantanen, V.; Hirvonen, H.; Kulmala, J.; Grènman, R.; Grènman, S. Effects of Paclitaxel With or Without Cremophor EL on Cellular Clonogenic Survival and Apoptosis. Eur. J. Cancer 1999, 35, 5. [Google Scholar] [CrossRef]
- Werle, M. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm. Res. 2008, 25, 500–511. [Google Scholar] [CrossRef]
- Guo, Y.; Chu, M.; Tan, S.; Zhao, S.; Liu, H.; Otieno, B.O.; Yang, X.; Xu, C.; Zhang, Z. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol. Pharm. 2014, 11, 59–70. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Chen, Y.; Ye, J.; Sha, X.; Fang, X. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Ford, J.M. Modulators of multidrug resistance: Preclinical studies. Hematol. Oncol. Clin. N. Am. 1995, 9, 337–362. [Google Scholar] [CrossRef]
- Qiu, L.; Qiao, M.; Chen, Q.; Tian, C.; Long, M.; Wang, M.; Li, Z.; Hu, W.; Li, G.; Cheng, L.; et al. Enhanced effect of pH-sensitive mixed copolymer micelles for overcoming multidrug resistance of doxorubicin. Biomaterials 2014, 35, 9877–9887. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Zhang, H.; Wang, Y.; Cheng, C.; Li, X.; Gu, C.; Yang, Q.; Wu, H.; Zhang, S. Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials 2010, 31, 5634–5642. [Google Scholar] [CrossRef]
- Duan, X.; Xiao, J.; Yin, Q.; Zhang, Z.; Yu, H.; Mao, S.; Li, Y. Smart pH-Sensitive and TemporalControlled Polymeric Micelles for Effective Combination Therapy of Doxorubicin and Disulfiram. ACS Nano 2013, 7, 12. [Google Scholar] [CrossRef]
- Guo, X.; Wei, X.; Jing, Y.; Zhou, S. Size changeable nanocarriers with nuclear targeting for effectively overcoming multidrug resistance in cancer therapy. Adv. Mater. 2015, 27, 6450–6456. [Google Scholar] [CrossRef]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X.; et al. pH-and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Chen, Z.S. Multidrug resistance proteins (MRPs) and cancer therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef]
- Jia, N.; Ye, Y.; Wang, Q.; Zhao, X.; Hu, H.; Chen, D.; Qiao, M. Preparation and evaluation of poly( l -histidine) based pH-sensitive micelles for intracellular delivery of doxorubicin against MCF-7/ADR cells. Asian J. Pharm. Sci. 2017, 12, 433–441. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Gao, Y.; Liu, Y.; Liu, J.; Zou, M.; Wang, L.; Cheng, G. Lipid-albumin nanoassemblies co-loaded with borneol and paclitaxel for intracellular drug delivery to C6 glioma cells with P-gp inhibition and its tumor targeting. Asian J. Pharm. Sci. 2015, 10, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 23. [Google Scholar] [CrossRef]
- Schneidera, Y.; Vincent, F.; Duranton, B.; Badolo, L.; Gossé, F.; Bergmann, C.; Seiler, N.; Raul, F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000, 158, 7. [Google Scholar] [CrossRef]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Ba, S.; Zhu, J.; Zhang, J.; Hong, W.; Zhao, X.; Hu, H.; Qiao, M. Poly(l-histidine) based triblock copolymers: pH induced reassembly of copolymer micelles and mechanism underlying endolysosomal escape for intracellular delivery. Biomacromolecules 2014, 15, 4032–4045. [Google Scholar] [CrossRef]
- Altreuter, D.H.; Dordick, J.S.; Clark, D.S. Nonaqueous Biocatalytic Synthesis of New Cytotoxic Doxorubicin Derivatives: Exploiting Unexpected Differences in the Regioselectivity of Salt Activated and Solubilized Subtilisin. J. Am. Chem. Soc. 2002, 124, 6. [Google Scholar] [CrossRef]
- Hao, T.; Chen, D.; Liu, K.; Qi, Y.; Tian, Y.; Sun, P.; Li, Y.; Li, Z. Micelles of d-alpha-Tocopheryl Polyethylene Glycol 2000 Succinate (TPGS 2K) for Doxorubicin Delivery with Reversal of Multidrug Resistance. ACS Appl. Mater. Interfaces 2015, 7, 18064–18075. [Google Scholar] [CrossRef]
- Hong, W.; Chen, D.; Jia, L.; Gu, J.; Hu, H.; Zhao, X.; Qiao, M. Thermo- and pH-responsive copolymers based on PLGA-PEG-PLGA and poly(L-histidine): Synthesis and in vitro characterization of copolymer micelles. Acta Biomater. 2014, 10, 1259–1271. [Google Scholar] [CrossRef]
- Assanhou, A.G.; Li, W.; Zhang, L.; Xue, L.; Kong, L.; Sun, H.; Mo, R.; Zhang, C. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials 2015, 73, 284–295. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Z.; Pan, S.; Shi, M.; Li, J.; Yang, C.; Hu, H.; Qiao, M.; Chen, D. Overcoming Multidrug Resistance by Codelivery of MDR1-Targeting siRNA and Doxorubicin Using EphA10-Mediated pH-Sensitive Lipoplexes: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 21590–21600. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, L.; Wang, Q.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. pH/Redox Dual-Responsive Polyplex with Effective Endosomal Escape for Codelivery of siRNA and Doxorubicin against Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. [Google Scholar] [CrossRef]
- Liang, D.; Wang, A.; Yang, Z.; Liu, Y.; Qi, X. Enhance Cancer Cell Recognition and Overcome Drug Resistance Using Hyaluronic Acid and alpha-Tocopheryl Succinate Based Multifunctional Nanoparticles. Mol. Pharm 2015, 12, 2189–2202. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Xie, J.; Qi, R.; Lu, H.; Leporatti, S.; Chen, J.; Hu, Y. pH-Sensitive Poly(beta-amino ester)s Nanocarriers Facilitate the Inhibition of Drug Resistance in Breast Cancer Cells. Nanomaterials 2018, 8, 952. [Google Scholar] [CrossRef]
- Zhu, J.; Qiao, M.; Wang, Q.; Ye, Y.; Ba, S.; Ma, J.; Hu, H.; Zhao, X.; Chen, D. Dual-responsive polyplexes with enhanced disassembly and endosomal escape for efficient delivery of siRNA. Biomaterials 2018, 162, 47–59. [Google Scholar] [CrossRef]
- Nicklisch, S.C.; Rees, S.D.; McGrath, A.P.; Gökirmak, T.; Bonito, L.T.; Vermeer, L.M.; Cregger, C.; Loewen, G.; Sandin, S.; Chang, G.; et al. Global marine pollutants inhibit P-glycoprotein: Environmental levels, inhibitory effects, and cocrystal structure. Sci. Adv. 2016, 2, e1600001. [Google Scholar] [CrossRef]
- McCormick, J.W.; Vogel, P.D.; Wise, J.G. Multiple drug transport pathways through human P-glycoprotein. Biochemistry 2015, 54, 4374–4390. [Google Scholar] [CrossRef]
- Dartier, J.; Lemaitre, E.; Chourpa, I.; Goupille, C.; Servais, S.; Chevalier, S.; Mahéo, K.; Dumas, J.K. ATP-dependent activity and mitochondrial localization of drug efflux pumps in doxorubicin-resistant breast cancer cells. Biochim. Et Biophys. Acta Gen. Subj. 2017, 1861, 1075–1084. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, E.O.; Ko, S.G.; Bae, H.S.; Kim, C.H.; Ahn, S.K.; Lu, J.; Kim, S.H. Mitochondria-cytochrome C-caspase-9 cascade mediates isorhamnetin-induced apoptosis. Cancer Lett. 2008, 270, 342–353. [Google Scholar] [CrossRef]
- St-Louis, M.C.; Archambault, D. The equine arteritis virus induces apoptosis via caspase-8 and mitochondria-dependent caspase-9 activation. Virology 2007, 367, 147–155. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamye, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.; Dou, H.J.; Fan, X.; Fei, X.C.; Wang, L.; Cheng, S.; Janin, A.; Wang, L.; Zhao, W.L. Doxorubicin-loaded dextran-based nano-carriers for highly efficient inhibition of lymphoma cell growth and synchronous reduction of cardiac toxicity. Int. J. Nanomed. 2018, 13, 5673. [Google Scholar] [CrossRef]
- Pawar, S.; Shevalkar, G.; Vavia, P. Glucosamine-anchored doxorubicin-loaded targeted nano-niosomes: Pharmacokinetic, toxicity and pharmacodynamic evaluation. J. Drug Target. 2016, 24, 730–743. [Google Scholar] [CrossRef]










| Formulation | Particle Size (nm) | EE% | DL% | ||
|---|---|---|---|---|---|
| Dox | Res | Dox | Res | ||
| pH-endoSM/Dox | 52.7 ± 0.22 | 95.2 ± 0.81 | - | 8.63 ± 0.22 | - |
| pH-endoSM/Dox/Res | 75.1 ± 0.32 | 80.2 ± 0.38 | 82.3 ± 0.71 | 6.92 ± 0.12 | 7.10 ± 0.20 |
| Formulation | IC50 | RF |
|---|---|---|
| Dox solution | 131.60 ± 4.12 | - |
| pH-endoSM/Dox | 36.30 ± 1.16 | 3.63 |
| pH-endoSM/Dox/Res | 7.57 ± 0.63 | 17.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Jia, N.; Gao, Y.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. Multi-Modulation of Doxorubicin Resistance in Breast Cancer Cells by Poly(l-histidine)-Based Multifunctional Micelles. Pharmaceutics 2019, 11, 385. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080385
Jia L, Jia N, Gao Y, Hu H, Zhao X, Chen D, Qiao M. Multi-Modulation of Doxorubicin Resistance in Breast Cancer Cells by Poly(l-histidine)-Based Multifunctional Micelles. Pharmaceutics. 2019; 11(8):385. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080385
Chicago/Turabian StyleJia, Li, Nan Jia, Yan Gao, Haiyang Hu, Xiuli Zhao, Dawei Chen, and Mingxi Qiao. 2019. "Multi-Modulation of Doxorubicin Resistance in Breast Cancer Cells by Poly(l-histidine)-Based Multifunctional Micelles" Pharmaceutics 11, no. 8: 385. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080385