Sustained Release of Minor-Groove-Binding Antibiotic Netropsin from Calcium-Coated Groove-Rich DNA Particles
Abstract
:1. Introduction
2. Materials and Methods
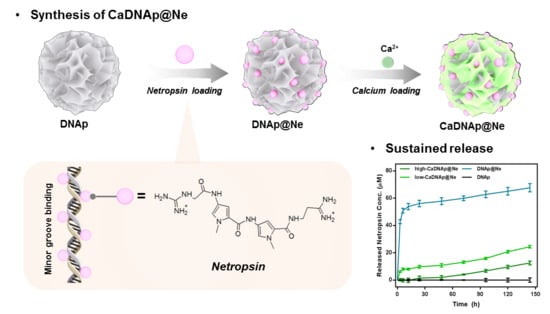
2.1. Overview of CaDNAp@Ne
2.2. Materials and Reagents
2.3. Fabrication of Circular DNA from Linear DNA
2.4. Synthesis of DNAp by RCA
2.5. Synthesis of DNAp@Ne
2.5.1. Synthesis of DNAp@Ne via Netropsin Loading
2.5.2. Stoichiometric Analysis of the Netropsin Loading Process
2.6. Synthesis of CaDNAp via Coating of Ca2+ on DNAp
2.6.1. Synthesis of CaDNAp via Ca2+ Coating on DNAp
2.6.2. Stoichiometric Analysis of the Ca2+ Coating Process
2.6.3. Characterization of CaDNAp
2.7. Netropsin Stoichiometric Release Analysis from CaDNAp@Ne
2.8. Viability Assay of CaDNAp@Ne
3. Results
3.1. Stoichiometric Analysis of the Netropsin Loading Process
3.2. Synthesis of DNAp@Ne via Netropsin Loading to DNAp
3.3. Synthesis and Characterization of CaDNAp
3.4. Evaluation of the Antibiotic Effects of CaDNAp@Ne
4. Discussions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Avery, O.T.; MacLeod, C.M.; McCarty, M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types. J. Exp. Med. 1944, 79, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.D.; Chase, M. Independent Functions of Viral Protein and Nucleic Acid in Growth of Bacteriophage. J. Gen. Physiol. 1952, 36, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Zhurkin, V.B.; Tolstorukov, M.Y.; Xu, F.; Colasanti, A.V.; Olson, W.K. Sequence-Dependent Variability of B-DNA. In DNA Conformation and Transcription; Ohyama, T., Ed.; Springer: Boston, MA, USA, 2005; Volume 1, pp. 18–34. [Google Scholar]
- Kim, J.Y.; Lee, J.S. Synthesis and Thermally Reversible Assembly of DNA-Gold Nanoparticle Cluster Conjugates. Nano Lett. 2009, 9, 4564–4569. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA Origami with Complex Curvatures in Three-Dimensional Space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, J.D.; Crick, F.H. Molecular Structure of Nucleic Acids. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. DNA in a Material World. Nature 2003, 421, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tseng, Y.D.; Kwon, S.Y.; D’esqaux, L.; Bunch, J.S.; Mceuen, P.L.; Luo, D. Controlled Assembly of Dendrimer-Like DNA. Nat. Mater. 2004, 3, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cu, Y.T.; Luo, D. Multiplexed Detection of Pathogen DNA with DNA-Based Fluorescence Nanobarcodes. Nat. Biotechnol. 2005, 23, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-Catalysed Assembly of DNA Hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Liu, H.; Ribbe, A.E.; Mao, C. Self-Assembly of Hexagonal DNA Two-Dimensional (2d) Arrays. J. Am. Chem. Soc. 2005, 127, 12202–12203. [Google Scholar] [CrossRef]
- Wang, P.; Wu, S.; Tian, C.; Yu, G.; Jiang, W.; Wang, G.; Mao, C. Retrosynthetic Analysis-Guided Breaking Tile Symmetry for the Assembly of Complex DNA Nanostructures. J. Am. Chem. Soc. 2016, 138, 13579–13585. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, R.; Ratanalert, S.; Zhang, K.; Zhang, F.; Yan, H.; Chiu, W.; Bathe, M. Designer Nanoscale DNA Assemblies Programmed from the Top Down. Science 2016, 352, 1534. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.; Mohammed, A.; Gardell, J.; Masich, S.; Czeizler, E.; Orponen, P.; Högberg, B. DNA Rendering of Polyhedral Meshes at the Nanoscale. Nature 2015, 523, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W. Folding DNA to Create Nanoscale Shapes and Patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-Assembly of DNA into Nanoscale Three-Dimensional Shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Douglas, S.M.; Liu, M.; Sharma, J.; Cheng, A.; Leung, A.; Liu, Y.; Shih, W.M.; Yan, H. Multilayer DNA Origami Packe on a Square Lattice. J. Am. Chem. Soc. 2009, 131, 15903–15908. [Google Scholar] [CrossRef]
- Han, D.; Qi, X.; Myhrvold, C.; Wang, B.; Dai, M.; Jiang, S.; Bates, M.; Liu, Y.; An, B.; Zhang, F.; et al. Single-Stranded DNA and Rna Origami. Science 2017, 358, eaao2648. [Google Scholar] [CrossRef]
- Ali, M.M.; Li, F.; Zhang, Z.; Zhang, K.; Kang, D.G.; Ankrum, J.A.; Le, X.C.; Zhao, W. Rolling Circle Amplification: A Versatile Tool for Chemical Biology, Materials Science and Medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef]
- Zhao, W.; Ali, M.M.; Brook, M.A.; Li, Y. Rolling Circle Amplification: Applications in Nanotechnology and Biodetection with Functional Nucleic Acids. Angew. Chem. Int. Ed. 2008, 47, 6330–6337. [Google Scholar] [CrossRef]
- Han, S.; Lee, J.S.; Lee, J.B. Synthesis of a Multi-Functional DNA Nanosphere Barcode System for Direct Cell Detection. Nanoscale 2017, 9, 14094–14102. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.; Jo, C.; Jeong, J.; Ko, J.; Han, S.; Lee, M.S.; Lee, H.Y.; Han, J.W.; Lee, J.; et al. Enzymatic-Driven Hasselback-Like DNA-Based Inorganic Superstructures. Adv. Funct. Mater. 2017, 27, 1704213. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, H.; Lee, D.J.; Jung, B.J.; Lee, J.B. Rca-Based Biosensor for Electrical and Colorimetric Detection of Pathogen DNA. Nanoscale Res. Lett. 2016, 11, 242. [Google Scholar] [CrossRef]
- Kim, E.; Zwi-Dantsis, L.; Reznikov, N.; Hansel, C.S.; Agarwal, S.; Stevens, M.M. One-Pot Synthesis of Multiple Protein-Encapsulated DNA Flowers and Their Application in Intracellular Protein Delivery. Adv. Mater. 2017, 29, 1701086. [Google Scholar] [CrossRef]
- Lee, J.B.; Peng, S.; Yang, D.; Roh, Y.H.; Funabashi, H.; Park, N.; Rice, E.J.; Chen, L.; Long, R.; Wu, M.; et al. A Mechanical Metamaterial Made from a DNA Hydrogel. Nat. Nanotechnol. 2012, 7, 816–820. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Liu, G.; Tian, L. A Pure DNA Hydrogel with Stable Catalytic Ability Produced by One-Step Rolling Circle Amplification. Chem. Commun. 2017, 53, 3038–3041. [Google Scholar] [CrossRef]
- Lerman, L.S. Structural Considerations in the Interaction of DNA and Acridines. J. Mol. Biol. 1961, 3, 18–30. [Google Scholar] [CrossRef]
- Ali, A.A.; Paramanathan, T.; Rouzina, I.; Williams, M.C. Mechanisms of Small Molecule–DNA Interactionsprobed by Single-Molecule Force Spectroscopy. Nucleic Acids Res. 2016, 44, 3971–3988. [Google Scholar]
- Mukherjee, A.; Sasikala, W.D. Chapter One-Drug-DNA Intercalation: From Discovery to the Molecular Mechanism. Adv. Protein Chem. Struct. Biol. 2013, 92, 1–62. [Google Scholar]
- Ulrich, S. 14. Metallointercalators and Metalloinsertors: Structural Requirements for DNA Recognition and Anticancer Activity. In Metallo-Drugs: Development and Action of Anticancer Agents; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K., Eds.; De Gruyter: Berlin, Germany, 2018; Volume 1. [Google Scholar]
- Biebricher, A.S.; Heller, I.; Roijmans, R.F.; Hoekstra, T.P.; Peterman, E.J.; Wuite, G.J. The Impact of DNA Intercalators on DNA and DNA-Processing Enzymes Elucidated through Force-Dependent Binding Kinetics. Nat. Commun. 2015, 6, 7304. [Google Scholar] [CrossRef]
- Jawad, B.; Pouldel, L.; Podgornik, R.; Steinmetz, N.F.; Ching, W. Molecular Mechanism and Binding Free Energy of Doxorubicin Intercalation in DNA. Phys. Chem. Chem. Phys. 2019, 21, 3877–3893. [Google Scholar] [CrossRef]
- Mišković, K.; Bujak, M.; Lončar, M.B.; Glavaš-Obrovac, L. Antineoplastic DNA-Binding Compounds: Intercalating and Minor Groove Binding Drugs. Arch. Ind. Hyg. Toxicol. 2013, 64, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Lauria, A.; Montalbano, A.; Barraja, P.; Dattolo, G.; Almerico, A.M. DNA Minor Groove Binders: An Overview on Molecular Modeling and Qsar Approaches. Curr. Med. Chem. 2007, 14, 2136–2160. [Google Scholar] [CrossRef]
- Khan, G.S.; Shah, A.; Rehman, Z.; Barker, D. Chemistry of DNA Minor Groove Binding Agents. J. Photochem. Photobiol. 2012, 115, 105–118. [Google Scholar] [CrossRef]
- Wierenga, W. DNA-Minor Groove Binding Anticancer Agents. In Cytotoxic Anticancer Drugs: Models and Concepts for Drug Discovery and Development; Developments in Oncology; Valeriote, F.A., Corbett, T.H., Baker, L.H., Eds.; Springer: Boston, MA, USA, 1992; Volume 68. [Google Scholar]
- Hamilton, P.L.; Arya, D.P. Natural Product DNA Major Groove Binders. Nat. Prod. Rep. 2012, 29, 134–143. [Google Scholar] [CrossRef]
- Paul, A.; Guo, P.; Boykin, D.W.; Wilson, W.D. A New Generation of Minor-Groove-Binding—Heterocyclic Diamidines That Recognize G·C Base Pairs in an at Sequence Context. Molecules 2019, 24, 946. [Google Scholar] [CrossRef]
- Huang, Q.; Baum, L.; Fu, W. Simple and Practical Staining of DNA with Gelred in Agarose Gel Electrophoresis. Clin. Lab. 2010, 56, 149–152. [Google Scholar]
- Brana, M.F.; Cacho, M.; Gradillas, A.; Pascual-Terasa, B.; Ramos, A. Intercalators as Anticancer Drugs. Curr. Pharm. Des. 2001, 7, 1745–1780. [Google Scholar] [CrossRef]
- Waring, M. Binding of Antibiotics to DNA. Ciba Found. Symp. 1991, 158, 128–142. [Google Scholar]
- Zhao, Y.; Shaw, A.; Zeng, X.; Benson, E.; Nyström, A.M.; Högberg, B. DNA Origami Delivery System for Cancer Therapy with Tunable Release Properties. ACS Nano 2012, 6, 8684–8691. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Xia, S.; Wu, K.; Huang, Z.; Chen, H.; Chen, J.; Zhang, J. A Ph/Enzyme-Responsive Tumor-Specific Delivery System for Doxorubicin. Biomaterials 2010, 31, 6309–6316. [Google Scholar] [CrossRef]
- Alemdaroglu, F.E.; Alemdaroglu, N.C.; Langguth, P.; Herrmann, A. DNA Block Copolymer Micelles–a Combinatorial Tool for Cancer Nanotechnology. Adv. Mater. 2008, 20, 899–902. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.B.; Hong, J. Controlled Release of an Anti-Cancer Drug from DNA Structured Nano-Films. Sci. Rep. 2014, 4, 4078. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, G.; Cho, S.J.; Geckeler, K.E.; Fuchs, H. Cellular Interactions of Doxorubicin-Loaded DNA-Modified Halloysite Nanotubes. Nanoscale 2013, 5, 8577–8585. [Google Scholar] [CrossRef]
- Bailly, C.; Chaires, J.B. Sequence-Specific DNA Minor Groove Binders: Design and Synthesis of Netropsin and Distamycin Analogues. Bioconj. Chem. 1998, 9, 513–538. [Google Scholar] [CrossRef]
- Jeon, H.; Han, S.; Kim, H.; Lee, J.B. Surface Modification of Rna Nanoparticles by Ionic Interaction for Efficient Cellular Uptake. J. Ind. Eng. Chem. 2019, 70, 87–93. [Google Scholar] [CrossRef]
- Danilevich, V.N.; Machulin, A.V.; Lipkin, A.V.; Kulakovskaya, T.V.; Smith, S.S.; Mulyukin, A.L. New Insight into Formation of DNA-Containing Microparticles During Pcr: The Scaffolding Role of Magnesium Pyrophosphate Crystals. J. Biomol. Struct. Dyn. 2016, 34, 625–639. [Google Scholar] [CrossRef]
- Danilevich, V.N.; Mulyukin, A.L.; Machulin, A.V.; Sorokin, V.V.; Kozlov, S.A. Structural Variability of DNA-Containing Mg-Pyrophosphate Microparticles: Optimized Conditions to Produce Particles with Desired Size and Morphology. J. Biomol. Struct. Dyn. 2019, 37, 918–930. [Google Scholar] [CrossRef]
- Lee, J.B.; Hong, J.K.; Bonner, D.K.; Poon, Z.; Hammond, P.T. Self-Assembled Rna Interference Microsponges for Efficient Sirna Delivery. Nat. Mater. 2012, 11, 316–322. [Google Scholar] [CrossRef]
- Trouet, A.; Campeneere, D.D.; Duve, C.D. Chemotherapy through Lysosomes with a DNA-Daunorubicin Complex. Nat. New Biol. 1972, 239, 110–112. [Google Scholar] [CrossRef]
- Majee, S.; Dasgupta, D.; Chakrabarti, A. Interaction of the DNA-Binding Antitumor Antibiotics, Chromomycin Andmithramycin with Erythroid Spectrin. Eur. J. Biochem. 1999, 260, 619–626. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.; Nam, H.; Lee, J.B. Sustained Release of Minor-Groove-Binding Antibiotic Netropsin from Calcium-Coated Groove-Rich DNA Particles. Pharmaceutics 2019, 11, 387. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080387
Jeon H, Nam H, Lee JB. Sustained Release of Minor-Groove-Binding Antibiotic Netropsin from Calcium-Coated Groove-Rich DNA Particles. Pharmaceutics. 2019; 11(8):387. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080387
Chicago/Turabian StyleJeon, Hyunsu, Hyangsu Nam, and Jong Bum Lee. 2019. "Sustained Release of Minor-Groove-Binding Antibiotic Netropsin from Calcium-Coated Groove-Rich DNA Particles" Pharmaceutics 11, no. 8: 387. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080387