Long-Term Physical (In)Stability of Spray-Dried Amorphous Drugs: Relationship with Glass-Forming Ability and Physicochemical Properties
Abstract
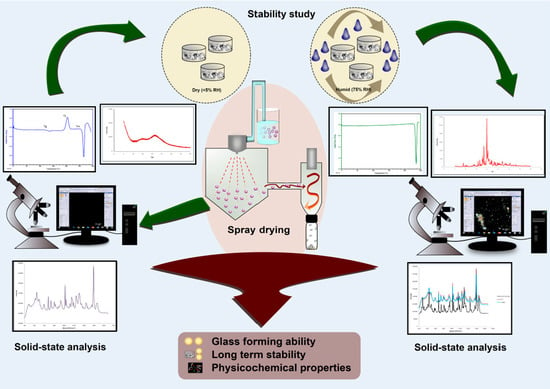
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.2.1. Melt-Quenching Using Differential Scanning Calorimetry
2.2.2. Spray-Drying
2.3. Physical Stability upon Storage
2.4. Solid-State Characterization
2.4.1. Powder X-ray Diffraction (PXRD)
2.4.2. Modulated Differential Scanning Calorimetry (MDSC)
Determination of Melting Point and Heat of Fusion
Solid-State Characteristics of Spray-Dried Stability Samples
Determination of GFA/GS Classes of Spray-Dried Samples
2.4.3. Raman Spectroscopy
2.4.4. Polarized Light Microscopy
2.5. Combining the PXRD, DSC, Raman Spectroscopy, and PLM Analyses
2.6. Univariate and Multivariate Analysis
3. Results and Discussion
3.1. Selection of the Dataset
3.2. Glass-Forming Ability and Glass Stability
3.2.1. Influence of Preparation Method vs. Compound GFA/GS
3.3. Long-Term Physical Stability
3.4. Relationship between GFA/GS Classifications and Storage Stability
3.5. Role of the Physicochemical Properties on the GFA and Long-Term Physical Stability
3.5.1. GFA vs. Physicochemical Properties
3.5.2. Long Term Physical Stability vs. Physicochemical Properties
3.5.3. Physicochemical Properties of Importance to GFA and Physical Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical Particle Technologies: An Approach to Improve Drug Solubility, Dissolution and Bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical Dispersion Techniques for Dissolution and Bioavailability Enhancement of Poorly Water-Soluble Drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Le, J.; Hersey, A.; Luscombe, C.N.; Beck, G.; Sherborne, B.; Cooper, I. Rate-Limited Steps of Human Oral Absorption and Qsar Studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Edueng, K.; Mahlin, D.; Bergström, C.A.S. The Need for Restructuring the Disordered Science of Amorphous Drug Formulations. Pharm. Res. 2017, 34, 1754–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, J.A.; van Eerdenbrugh, B.; Taylor, L.S. A Classification System to Assess the Crystallization Tendency of Organic Molecules from Undercooled Melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Glass Forming Ability of Amorphous Drugs Investigated by Continuous Cooling and Isothermal Transformation. Mol. Pharm. 2016, 13, 3318–3325. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Baird, J.A.; Taylor, L.S. Crystallization Tendency of Active Pharmaceutical Ingredients Following Rapid Solvent Evaporation—Classification and Comparison with Crystallization Tendency from under Cooled Melts. J. Pharm. Sci. 2010, 99, 3826–3838. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Alzghoul, A.; Kaialy, W.; Mahlin, D.; Bergstrom, C.A. Computational Predictions of Glass-Forming Ability and Crystallization Tendency of Drug Molecules. Mol. Pharm. 2014, 11, 3123–3132. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Alzghoul, A.; Mahlin, D.; Bergström, C.A.S. Physical Stability of Drugs after Storage above and Below the Glass Transition Temperature: Relationship to Glass-Forming Ability. Int. J. Pharm. 2015, 495, 312–317. [Google Scholar] [CrossRef]
- Mahlin, D.; Bergström, C.A.S. Early Drug Development Predictions of Glass-Forming Ability and Physical Stability of Drugs. Eur. J. Pharm. Sci. 2013, 49, 323–332. [Google Scholar] [CrossRef]
- Mahlin, D.; Ponnambalam, S.; Höckerfelt, M.H.; Bergström, C.A.S. Toward in Silico Prediction of Glass-Forming Ability from Molecular Structure Alone: A Screening Tool in Early Drug Development. Mol. Pharm. 2011, 8, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kuentz, M.; Holm, R.; Elder, D.P. Methodology of Oral Formulation Selection in the Pharmaceutical Industry. Eur. J. Pharm. Sci. 2016, 87, 136–163. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Lindenberg, E.; Rades, T.; Grohganz, H.; Löbmann, K. Influence of Preparation Pathway on the Glass Forming Ability. Int. J. Pharm. 2017, 521, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Graeser, K.A.; Strachan, C.J.; Patterson, J.E.; Gordon, K.C.; Rades, T. Physicochemical Properties and Stability of Two Differently Prepared Amorphous Forms of Simvastatin. Cryst. Growth Des. 2008, 8, 128–135. [Google Scholar] [CrossRef]
- Karmwar, P.; Graeser, K.; Gordon, K.C.; Strachan, C.J.; Rades, T. Investigation of Properties and Recrystallisation Behaviour of Amorphous Indomethacin Samples Prepared by Different Methods. Int. J. Pharm. 2011, 417, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, N.; Kuentz, M. Glass-Forming Ability of Compounds in Marketed Amorphous Drug Products. Eur. J. Pharm. Biopharm. 2017, 112, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Willart, J.F.; Descamps, M. Solid State Amorphization of Pharmaceuticals. Mol. Pharm. 2008, 5, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Edueng, K.; Mahlin, D.; Larsson, P.; Bergstrom, C.A.S. Mechanism-Based Selection of Stabilization Strategy for Amorphous Formulations: Insights into Crystallization Pathways. J. Control. Release 2017, 256, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. Effect of Water on Molecular Mobility and Physical Stability of Amorphous Pharmaceuticals. Mol. Pharm. 2016, 13, 1339–1346. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. The Relationship between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Pazesh, S.; Lazorova, L.; Berggren, J.; Alderborn, G.; Grasjo, J. Considerations on the Quantitative Analysis of Apparent Amorphicity of Milled Lactose by Raman Spectroscopy. Int. J. Pharm. 2016, 511, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Nurzynska, K.; Booth, J.; Roberts, C.J.; McCabe, J.; Dryden, I.; Fischer, P.M. Long-Term Amorphous Drug Stability Predictions Using Easily Calculated, Predicted, and Measured Parameters. Mol. Pharm. 2015, 12, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Mah, P.T.; Laaksonen, T.; Rades, T.; Aaltonen, J.; Peltonen, L.; Strachan, C.J. Unravelling the Relationship between Degree of Disorder and the Dissolution Behavior of Milled Glibenclamide. Mol. Pharm. 2014, 11, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Nishizawa, J.-I.; Fukura, N.; Sasaki, T. Characterization of Poly-Amorphous Indomethacin by Terahertz Spectroscopy. J. Infrared Millim. Terahertz Waves 2012, 33, 953–962. [Google Scholar] [CrossRef]
- Lefort, R.; de Gusseme, A.; Willart, J.F.; Danède, F.; Descamps, M. Solid State Nmr and Dsc Methods for Quantifying the Amorphous Content in Solid Dosage Forms: An Application to Ball-Milling of Trehalose. Int. J. Pharm. 2004, 280, 209–219. [Google Scholar] [CrossRef]
- Bøtker, J.P.; Karmwar, P.; Strachan, C.J.; Cornett, C.; Tian, F.; Zujovic, Z.; Rantanen, J.; Rades, T. Assessment of Crystalline Disorder in Cryo-Milled Samples of Indomethacin Using Atomic Pair-Wise Distribution Functions. Int. J. Pharm. 2011, 417, 112–119. [Google Scholar] [CrossRef]
- Savolainen, M.; Heinz, A.; Strachan, C.; Gordon, K.C.; Yliruusi, J.; Rades, T.; Sandler, N. Screening for Differences in the Amorphous State of Indomethacin Using Multivariate Visualization. Eur. J. Pharm. Sci. 2007, 30, 113–123. [Google Scholar] [CrossRef]
- Kilburn, D.; Townrow, S.; Meunier, V.; Richardson, R.; Alam, A.; Ubbink, J. Organization and Mobility of Water in Amorphous and Crystalline Trehalose. Nat. Mater. 2006, 5, 632–635. [Google Scholar] [CrossRef]
- Kauzmann, W. The Nature of the Glassy State and the Behavior of Liquids at Low Temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Turnbull, D. Under What Conditions Can a Glass Be Formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Baird, J.A.; Santiago-Quinonez, D.; Rinaldi, C.; Taylor, L.S. Role of Viscosity in Influencing the Glass-Forming Ability of Organic Molecules from the Undercooled Melt State. Pharm. Res. 2012, 29, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, N.; Kirchmeyer, W.; Alsenz, J.; Kuentz, M. Theoretical Considerations of the Prigogine–Defay Ratio with Regard to the Glass-Forming Ability of Drugs from Undercooled Melts. Mol. Pharm. 2016, 13, 241–250. [Google Scholar] [CrossRef] [PubMed]







| Compound | GFA MQ | GFASD | MW (g/mol) | logP | HBD | HBA | RotB | PSA (Å2) | Tg MQ (°C) a | Tg SD (°C) b | Tc MQ (°C) c | Tc SD (°C) d | Tm (°C) | Trg | ΔHf (J/g) | ΔSf (Jg−1K−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetaminophen | II | I | 151 | 0.5 | 2 | 2 | 1 | 49 | 27 | n.d | 78 | n.d | 166 | 0.16 | 206 | 0.47 |
| Aripiprazole | II | II | 448 | 4.6 | 1 | 4 | 7 | 45 | 37 | 31 | 91 | 63 | 136 | 0.27 | 92 | 0.22 |
| Bezafibrate | III | I | 362 | 3.8 | 2 | 4 | 7 | 76 | 42 | n.d | n.d. | n.d | 182 | 0.23 | 145 | 0.32 |
| Chlorpropamide | II | I | 277 | 2.3 | 2 | 3 | 4 | 84 | 19 | n.d | 62 | n.d | 127 | 0.15 | 101 | 0.25 |
| Cinnarizine | II | I | 369 | 5.8 | 0 | 2 | 6 | 7 | 10 | n.d | 78 | n.d | 117 | 0.09 | 118 | 0.30 |
| Clofoctol | II | I | 365 | 8.1 | 1 | 1 | 5 | 20 | −1 | n.d | 48 | n.d | 85 | −0.01 | 98 | 0.27 |
| Clotrimazole | III | II | 345 | 5 | 0 | 1 | 4 | 18 | 33 | 15 | n.d. | 88 | 141 | 0.23 | 101 | 0.24 |
| Droperidol | II | II | 379 | 3.5 | 1 | 4 | 6 | 53 | 36 | 33 | 117 | 73 | 149 | 0.24 | 103 | 0.24 |
| d-salicin | II | I | 286 | −1.2 | 5 | 7 | 4 | 120 | 62 | n.d | 129 | n.d | 198 | 0.31 | 201 | 0.43 |
| Fenofibrate | III | I | 361 | 5.2 | 0 | 4 | 7 | 53 | −16 | n.d | n.d. | n.d | 79 | −0.20 | 98 | 0.28 |
| Flurbiprofen | II | I | 244 | 4.2 | 1 | 3 | 3 | 37 | −2 | n.d | 38 | n.d | 111 | −0.02 | 116 | 0.30 |
| Glibenclamide | III | II | 494 | 4.8 | 3 | 5 | 8 | 122 | 61 | 75 | n.d. | 130 | 171 | 0.36 | 119 | 0.27 |
| Glipizide | II | II | 446 | 1.9 | 3 | 6 | 7 | 139 | 62 | 64 | 112 | 68 | 200 | 0.31 | 208 | 0.44 |
| Hydrochlorothiazide | III | II | 298 | −0.1 | 3 | 7 | 1 | 135 | 119 | 76 | n.d. | 125 | 264 | 0.45 | 122 | 0.23 |
| Hydrocortisone | III | II | 362 | 1.6 | 3 | 5 | 2 | 95 | 93 | 96 | n.d. | 137 | 216 | 0.43 | 121 | 0.25 |
| Ibuprofen | III | I | 206 | 3.5 | 1 | 2 | 4 | 37 | −40 | n.d | n.d. | n.d | 73 | −0.55 | 135 | 0.39 |
| Indapamide | III | III | 366 | 2.9 | 2 | 5 | 3 | 101 | 107 | 106 | n.d. | n.d | 163 | 0.66 | 74 | 0.17 |
| Ketoconazole | III | II | 531 | 4.3 | 0 | 6 | 7 | 69 | 48 | 43 | n.d. | 104 | 145 | 0.33 | 103 | 0.25 |
| Ketoprofen | III | I | 254 | 3.1 | 1 | 3 | 4 | 54 | 0 | n.d | n.d. | n.d | 91 | −0.01 | 114 | 0.31 |
| Metolazone | III | II | 366 | 4.1 | 2 | 5 | 2 | 101 | 121 | 137 | n.d. | 194 | 261 | 0.46 | 96 | 0.18 |
| Prednisone | III | II | 358 | 1.5 | 2 | 5 | 2 | 92 | 137 | 96 | n.d. | 92 | 186 | 0.74 | 95 | 0.21 |
| Probucol | III | II | 517 | 11.3 | 2 | 4 | 8 | 91 | 29 | 26 | n.d. | 44 | 123 | 0.24 | 68 | 0.17 |
| Procaine | III | I | 236 | 1.9 | 1 | 4 | 7 | 56 | −36 | n.d | n.d | n.d | 58 | −0.62 | 110 | 0.33 |
| Sulfamerazine | III | I | 264 | 0.1 | 2 | 6 | 3 | 106 | 69 | n.d | n.d. | n.d | 233 | 0.30 | 154 | 0.30 |
| Sulfathiazole | III | II | 255 | 0.1 | 2 | 6 | 3 | 122 | 65 | 51 | n.d. | 71 | 197 | 0.33 | 117 | 0.25 |
| Tinidazole | II | I | 247 | −0.4 | 0 | 5 | 4 | 106 | −7 | n.d | 53 | n.d | 123 | −0.06 | 145 | 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edueng, K.; Bergström, C.A.S.; Gråsjö, J.; Mahlin, D. Long-Term Physical (In)Stability of Spray-Dried Amorphous Drugs: Relationship with Glass-Forming Ability and Physicochemical Properties. Pharmaceutics 2019, 11, 425. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11090425
Edueng K, Bergström CAS, Gråsjö J, Mahlin D. Long-Term Physical (In)Stability of Spray-Dried Amorphous Drugs: Relationship with Glass-Forming Ability and Physicochemical Properties. Pharmaceutics. 2019; 11(9):425. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11090425
Chicago/Turabian StyleEdueng, Khadijah, Christel A.S. Bergström, Johan Gråsjö, and Denny Mahlin. 2019. "Long-Term Physical (In)Stability of Spray-Dried Amorphous Drugs: Relationship with Glass-Forming Ability and Physicochemical Properties" Pharmaceutics 11, no. 9: 425. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11090425